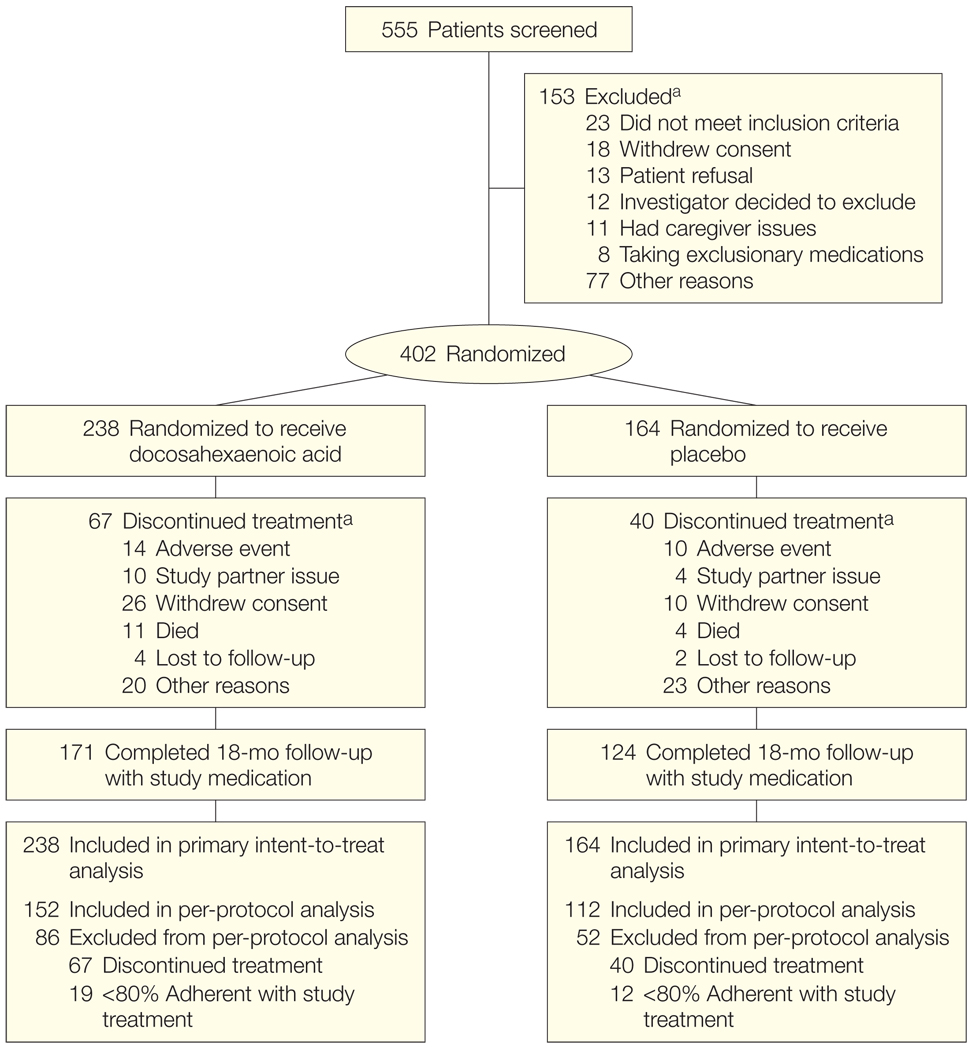
Figure 1.
Flow of Patients in the Alzheimer’s Disease Cooperative Study (ADCS) Docosahexaenoic Acid Supplementation Trial
Study partner issue is included as a reason for discontinuation because of the requirement for a study partner to participate in several of the key outcome measures in this trial (eg, Clinical Dementia Rating sum of boxes, ADCS activities of daily living, and Neuropsychiatric Inventory). There were no significant differences in incidence of dropout, adverse events, or serious adverse events (Table 2).
aThere could be more than 1 reason for exclusion or study discontinuation.