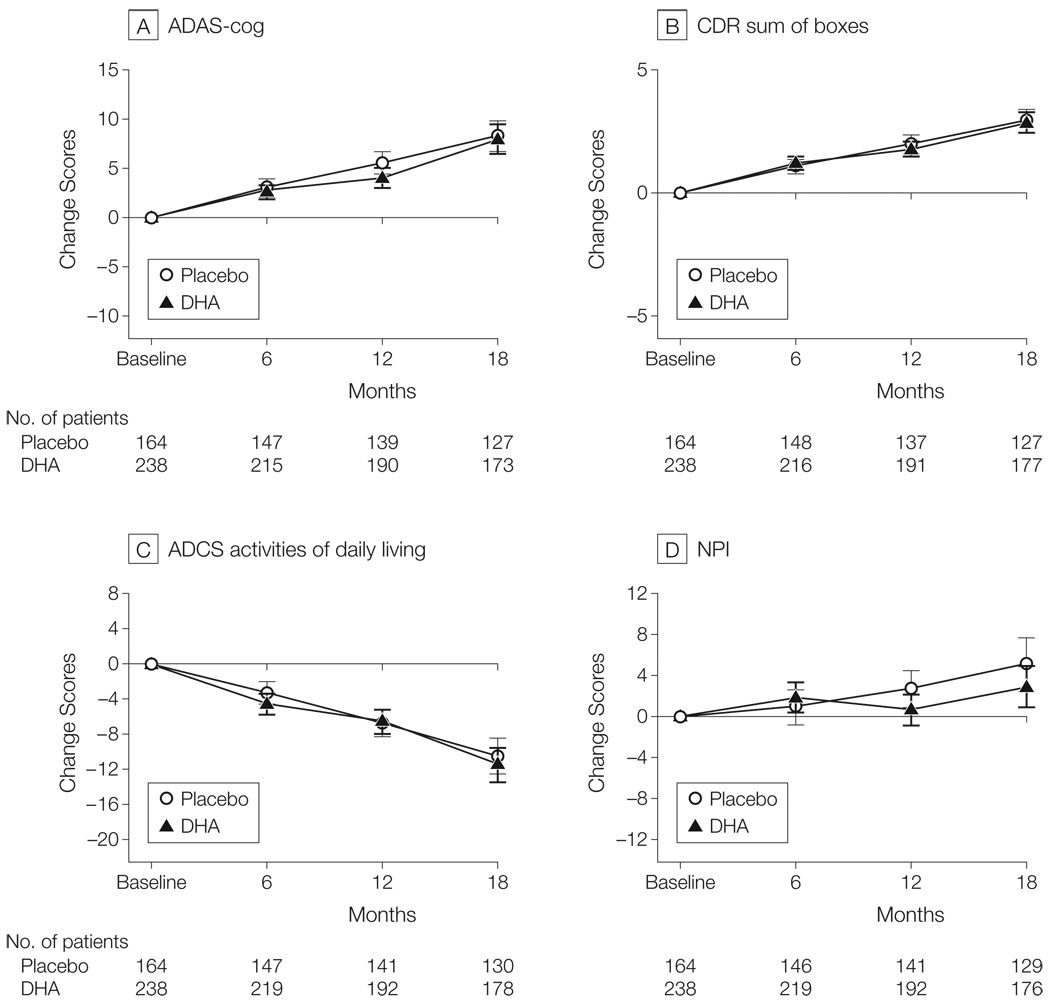
Figure 2.
Change in Primary and Secondary Outcome Measures in the Alzheimer’s Disease Cooperative Study (ADCS) Docosahexaenoic Acid (DHA) Supplementation Trial
All randomized participants were included in these intention-to-treat analyses. Error bars indicate 95% confidence intervals. There was no effect of DHA on rate of cognitive change on the Alzheimer’s Disease Assessment Scale (ADAS; linear mixed-effects model: P = .41), Clinical Dementia Rating (CDR) sum of boxes (linear mixed-effects model: P = .68), ADCS activities of daily living scale (linear mixed-effects model: P = .38), or Neuropsychiatric Inventory (NPI; linear mixed-effects model: P = .11). Scores for the ADAS-cog and CDR sum of boxes were the prespecified primary outcome measures; others were secondary outcomes.