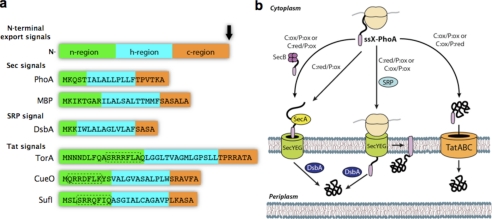
FIGURE 1.
Translocation of proteins through the inner membrane of bacteria. a, composition of N-terminally fused signal peptides used in this study. Each is composed of three linearly arranged domains: an n-region, a hydrophobic h-region, and a c-region that is followed by the signal peptide cleavage site (black arrow). The targeting specificity of Sec- and SRP-dependent signals is governed by the relative hydrophobicity of the h-region; SRP binds preferentially to the more hydrophobic signals found on SRP substrates. By comparison, Tat signals are typically about 15 amino acids longer than their Sec or SRP counterparts and contain a less hydrophobic h-region that appears to aid in targeting specificity. In addition, E. coli Tat signals possess a consensus motif (underlined) of (S/T)RRXFLK (where X is any polar amino acid) that is absent in Sec- and SRP-dependent signals. Further specificity for the Tat pathway is imparted by the occurrence of an overall charge of +2 or greater for the c-region together with the N terminus of the mature protein, as opposed to the typically neutral charge found in the c-region of Sec and SRP signal peptides. b, schematic of pathway specificity in the context of cellular redox potential. Export of PhoA (alkaline phosphatase) depends on the targeting specificity of the signal peptide (ssX), which includes (from left to right) SecB-dependent, SecB-independent, SRP-dependent, and Tat-dependent export as well as the relative redox potential (oxidizing (ox) or reducing (red)) of the cytoplasm (C) and periplasm (P).