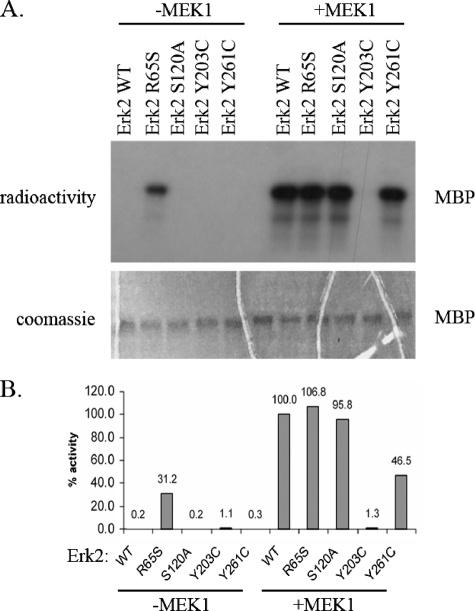
FIGURE 4.
ERK2R65S mutant is catalytically active in vitro. The Erk2 mutants, purified from E. coli, were treated (+MEK1) or not treated (–MEK1) with active MEK1 and ATP. Then, they were subjected to a standard kinase assay with MBP as a substrate. A, a fixed volume from each reaction was subjected to SDS-PAGE. Coomassie Brilliant Blue staining verified that equal amounts of substrate were loaded (lower panel). Then, the gel was exposed to x-ray film (upper panel). Note that the Erk2R65S variant is active independently of MEK1 phosphorylation. B, using the paper-spotted kinase assay technique, we quantified and normalized the activities of the mutants to that of the MEK1-activated Erk2WT that was defined as 100%. The Erk2R65S exhibited 30% activity relative to active Erk2WT. Results shown are the average of two independent experiments, each performed in triplicates.