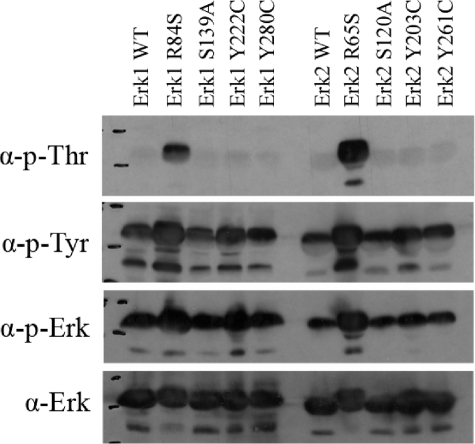
FIGURE 5.
Active variants of Erks are spontaneously phosphorylated in vitro on both Thr and Tyr residues. The recombinant purified wild-type and active mutants of Erks were subjected to a Western blot analysis. Antibodies that recognize the dually phosphorylated form of ERK1/2 (p-ERK), the phosphorylated-Tyr residues (p-Tyr), or the phosphorylated-Thr residues (p-Thr) were used. Antibody against ERK1/2 (α-ERK) was applied as well. It is apparent that all proteins are equally phosphorylated on Tyr, but the active mutants, Erk1R84S and Erk2R65S, were also phosphorylated on Thr at a very high level.