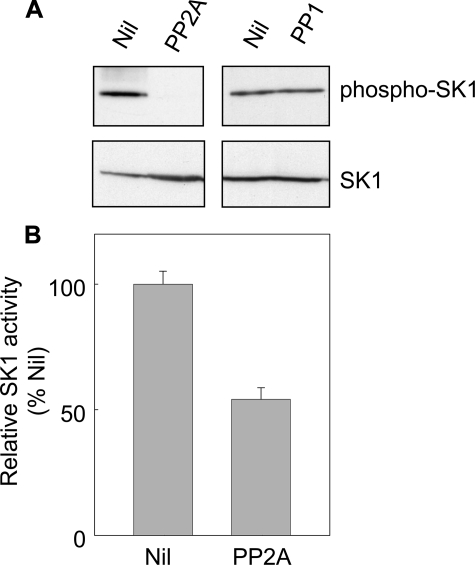
FIGURE 3.
PP2A, but not PP1, promoted dephosphorylation of SK1 in vitro. A, purified recombinant SK1 (1 μg) was incubated at 37 °C for 30 min in phosphatase buffer with 1 unit of commercial PP2A or PP1. 32P-labeled phosphorylase a was used as a control substrate to standardize the activities of the commercial PP1 and PP2A phosphatases. One unit of phosphatase activity is defined as the amount required to release 1 nmol of 32P/minute from phosphorylase a. Dephosphorylation of SK1 was assessed by immunoblotting with an antibody recognizing phospho-SK1 (upper panel) and total SK1 levels were confirmed by immunoblotting with an anti-His antibody (lower panel). B, relative catalytic activity of SK1 in the untreated versus PP2A-treated extract. Data are mean (±S.D.) from three independent experiments.