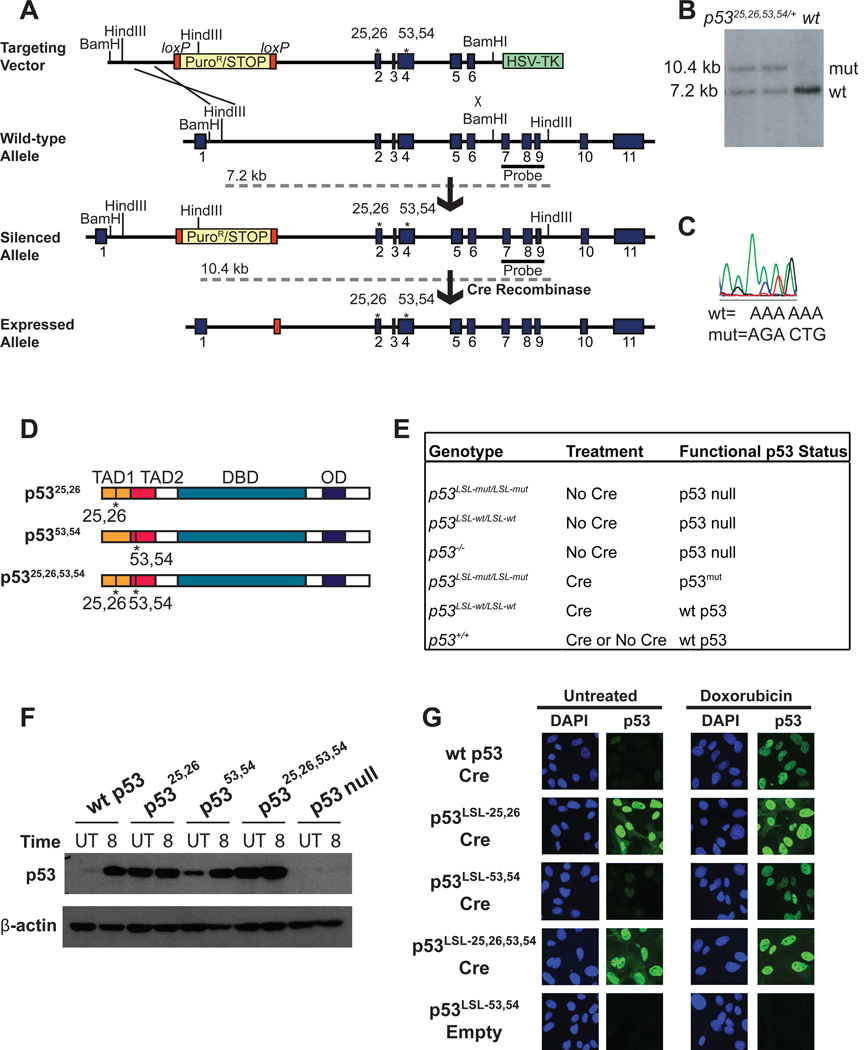
Fig 1. Generation of p53 TAD mutant knock-in mice.
(A) Targeting scheme used to generate knock-in mice, with the p5325,26,53,54 mutant as an example. Mutant p53 expression from targeted alleles is silenced until Cre introduction allows for excision of the stop element. Dotted grey lines indicate the sizes of the fragments generated from each allele upon HindIII digestion. (B) Southern blot showing 2 correctly targeted, heterozygous ES cell clones compared to a wild-type cell line. Genomic DNA was digested with HindIII and the Southern blot probed with the 3’ external probe indicated in (A). (C) Sequencing analysis of the reverse complement confirms the presence of point mutations in properly targeted cell lines. (D) Schematic of p5325,26, p5353,54, and p5325,26,53,54 proteins showing the transactivation domains (TADs), DNA binding domain (DBD), and oligomerization domain (OD). (E) Table summarizing the genotype, treatment, and ultimate functional p53 status of samples used throughout the manuscript. LSL-mut denotes any of the Lox-Stop-Lox p53 TAD mutants. (F) Western blot analysis for p53 in wild-type or homozygous p53LSL-mut MEFs transduced with Ad-Cre or Ad-empty (indicated as p53 null), either left untreated (UT) or treated for 8 hrs with 0.2 µg/ml dox (doxorubicin) (8). β-actin served as a loading control. Comparable, high efficiency (>90% of cells) of Cre recombination was confirmed by immunostaining for p53. (G) Immunofluorescence for p53 in wild-type or homozygous p53LSL-mut MEFs transduced with Ad-Cre or Ad-empty. Cells were left untreated (left) or treated with 0.2 µg/ml dox to stabilize p53 (right). Nuclei were stained with DAPI.