Abstract
Despite observational evidence of carry-over effects (COEs, events occurring in one season that produce residual effects on individuals the following seasons), to our knowledge no experimental studies have been carried out to explore how COEs might affect reproductive output. We simulated an environmental perturbation affecting spring-staging migrants to investigate COEs in greater snow geese (Anser caerulescens atlanticus). During three consecutive years, 2037 females captured during spring staging (approx. 3000 km south of their Arctic breeding grounds) were maintained in captivity (with or without access to food) for 0–4 days. Duration of captivity (but not food treatment) negatively affected reproductive success, probably through stress response. Reproductive success was reduced by 45–71% in 2 years, but not in a third year with unusually favourable breeding conditions. This unprecedented manipulation indicates that COEs can have a strong effect on individual reproductive success in long-distance migrants, but that this effect can be partly compensated for by good environmental conditions on the breeding ground.
Keywords: bird migration, carry-over effects, compensation, snow geese
1. Introduction
Migratory animals use several distinct sites over their annual cycle, and their population dynamics may therefore be affected by habitat quality encountered in these different areas [1,2]. Because of difficulties associated with tracking migrants across seasons and distant sites, identifying linkages between specific habitats and crucial parts of the life cycle that could limit population abundance is challenging [1–4]. Conditions encountered during breeding may often be a primary driver of population dynamics [5], but evidence is accumulating that conditions experienced during migration may be equally important [1,4]. Carry-over effects (COEs), i.e. events occurring in one season that produce residual effects on individuals the following seasons, can have large impacts on the population dynamics of migrants (see [4]). COEs are considered both from a theoretical and empirical perspective as crucial issues for a better understanding and modelling of a population's dynamic [4,6,7].
Beyond a desire to improve our understanding of migration ecology, there is an urgent need to quantify how rapidly changing conditions at sites used by migrants subsequently affect individual performance [4]. In a global change context, unpredictable events outside the reproductive period such as food shortages (e.g. spring snow storms, droughts, flooding, etc.), habitat loss and human disturbance (e.g. hunting, scaring of individuals from agricultural or recreational lands) are likely to increase [8,9]. Any of these events can potentially alter the nutrient demand or energetic state of organisms or induce a physiological stress [10], and thus can lead to COEs.
Despite observational evidence of COEs on some reproductive traits (condition, arrival date and laying date) of migrants [3,11–14], to our knowledge no experimental studies has yet been carried out. Studds & Marra [15] showed that individuals upgraded (i.e. those that maintained their body mass compared with controls) by a removal experiment were able to depart earlier on migration and had greater return rates the following winter than controls. This study demonstrated the impact of winter habitat occupancy on migratory performance though not directly on reproductive performance. Therefore, experimental demonstrations of COEs on reproductive performance at the individual level are still absent.
We used Arctic-nesting geese as a study model to experimentally test for such COEs. Geese are a suitable model because they depend in part on imported energy reserves to invest in egg production and incubation [16], and spring body condition is thought to be a key factor determining their reproductive output [16–18]. Examples of previously documented COEs negatively affecting reproduction in geese include scaring off agricultural lands in pink-footed geese (Anser brachyrhynchus; [19]) and non-lethal effects of hunting [8] in greater snow geese (Anser caerulescens atlanticus; [3]). In the latter example, disturbance owing to a new spring hunt reduced feeding time, increased flying time and precluded access to some foraging areas, which reduced nutrient storage [20] and reproductive success [21]. Such quasi-experimental conditions (unplanned, large-scale manipulation of the population) allowed examination of potential COEs at the population level, but not at the individual level and without the added benefit of random experimental design.
Our main goal was to experimentally mimic an environmental perturbation affecting spring body stores and stress levels [10] of migrants in order to test the hypothesis that changes in individual state during spring staging depressed their subsequent reproductive success. During three consecutive years, we captured and marked greater snow goose females at their main spring staging area. We maintained a sample of females and their mate in captivity for a variable amount of time (0–4 days) under different feeding conditions (with or without access to food) and released them before the massive departure of the goose population towards the arctic breeding grounds. We then determined the effect of our treatments on individual breeding success, defined as the probability of returning with young in the subsequent autumn. We predicted that fasting females should have a lower breeding success than captive-fed ones because they should experience a reduction of their body stores proportional to the length of their fast. Second, we predicted that control birds (i.e. those released immediately after capture) should have a higher breeding success than captive birds regardless of the feeding treatment owing to short- and long-term deleterious effects associated with the stress of captivity.
2. Material and methods
(a). Study site and species
Greater snow geese winter along the Atlantic coast of the United States and breed in the Canadian Arctic archipelago in Eastern and Northern Nunavut [22]. Twice a year, geese stage along the St Lawrence Valley of Quebec for six to eight weeks (late March to mid-late May in spring, late September to late November in autumn). This is their most important spring staging area where almost all their endogenous reserves are accumulated for the northward migration and subsequent reproduction [23]. During spring staging, geese feed both in tidal marshes along the St Lawrence River and in adjacent farmlands [20,22]. Geese were captured at Ile aux Oies (47°00′ N 70°33′ W), an island located in the St Lawrence estuary, 60 km northeast of Québec City (Canada). This site is an important staging site for migrating greater snow geese [20] and individual departure dates are relatively synchronized within a year, with virtually all birds leaving over an 8 day period in mid-late May [13]. Geese maintain permanent, monogamous pair bonds and remain in tight family groups (i.e. young of the year with their parents) for up to a year (i.e. until the following spring; [24]). Thus, a measure of individual reproductive success can be obtained in autumn and winter when the presence of juveniles with the parents can be ascertained through behavioural observations of individually identifiable geese.
(b). Capture of birds
From late April to mid May in 2007, 2008 and 2009, we captured geese using baited canon-nets. Sample sizes of captured birds are provided in table 1. We released juveniles immediately after capture (the spring migration is the period of normal family break-up in greater snow geese [25]). Adults were sexed based on cloacal examination. Females were weighed to the nearest gram with an electronic balance and culmen, head and tarsus were measured with a calliper to the nearest 0.1 mm shortly after capture. Females were individually marked with neck bands [26] and either released immediately after banding (typically less than 5 h after capture; control birds) with their mates or assigned to treatment groups with their mates (see details below). A capture group was assigned to a treatment in a pre-ordered list, i.e. first capture group = control; second capture = 2 days of fasting, etc. to ensure a balance design and minimize difference in sample size among treatments. The number of geese in a capture group did not influence the type of treatment.
Table 1.
Sample sizes of greater snow geese captured during spring staging, 2007–2009. (Captured groups were assigned as follows: control (mark and release immediately after capture), or kept from 2 to 4 days with ad libitum food (fed treatment) or without food (fasting treatment). The number of females resighted during the following autumn is split among the total resighted and those for which the family status was ascertained.)
| n capture groups | n females marked | control | fed treatment |
fasting treatment |
n females resighted | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| number of days |
number of days |
|||||||||
| 2 | 3 | 4 | 2 | 3 | 4 | |||||
| 2007 | 19 | 715 | 266 | 87 | 68 | 36 | 164 | 49 | 45 | 341/158 |
| 2008 | 20 | 649 | 209 | 125 | 40 | 62 | 142 | 40 | 31 | 299/221 |
| 2009 | 22 | 673 | 196 | 96 | 84 | 83 | 76 | 45 | 93 | 447/261 |
To assess whether body mass was a good index of body condition (i.e. fat), we benefited from females killed during captures in mid-May in 2006, 2007 and 2008. These females were dissected in the field immediately after collection and wet abdominal fat was weighed following Feret et al. [27].
(c). Experimental set-up
At the capture site, we maintained geese within eight fenced indoor enclosures. Fresh wheat straw was changed every 2 days in each enclosure. Water and food (where applicable) were refilled every morning and evening. In order to minimize the risk of pair bond breakage, all adults from the same capture group were kept in the barn and released at the same time, at the end of the treatment period, to allow them to reunite quickly. Male and females were kept in separate enclosures, but had social (visual and vocal) contacts. Because we aimed to manipulate only female condition, ad libitum access to food (crushed corn) and water was provided to all males. As found for females in the fed treatment, fed males should have maintained their body mass (see §3). All females from a given capture were either assigned to a ‘control group’ (released immediately with their mate; see above) or to one of the following feeding treatments: (i) fed groups were kept in enclosures with ad libitum food and water as in males, and (ii) fasting groups were kept in enclosure with only water. Treatment groups were released after 2, 3 or 4 days in captivity (table 1) and females were reweighed before release. Each year, the operations (captures and releases) ceased on 18th May; i.e. one week before the massive geese departure from the staging site.
(d). Determination of reproductive success
In October and November of each year, intensive resightings of marked geese were carried out by three experienced observers in areas where flocks of autumn staging birds were concentrated along the St Lawrence estuary [26]. For each marked female, observers sought to record the presence, absence and the number of young (table 1). The precise number of young per family group was difficult to ascertain in many cases owing to movements of families within large flocks. Moreover, geese are hunted in autumn [26] and the number of young per female may vary over the season [28]. Therefore, we used the presence or absence of young rather than the actual number of young because we felt that it was a more robust and less-biased index of reproductive success. Reed [29] showed that the probability of assigning a wrong family status in these conditions was low (less than 3%) in greater snow geese.
(e). Corrected body mass estimate
We corrected body mass for variation in body size. We first performed a principal component (PC) analysis on morphometric measurements (culmen, tarsus and head lengths). The three variables had loadings ranging from 0.50 to 0.63 on the first axis (PC1), which explained 73 per cent of total variation in the data. We used individual PC1 scores as a measure of body size. Because body mass steadily increased during spring (β = 11.6 ± 0.8 g d−1 (s.e.); F1,2043 = 187; p < 0.001), we included date when developing the corrected body mass index. Corrected body mass estimates were thus defined as the residuals of the regression of body mass on PC1 and date in Julian days (F2,2042 = 517.2; p < 0.001; adjusted r2 = 0.34) plus the mean mass of all individuals included in the model (we added the latter value solely for scaling purpose [27]). Corrected body mass (hereafter body mass) was positively related to abdominal fat (F1,89 = 56.19; p < 0.001; adjusted r2 = 0.39).
(f). Environmental conditions
Recently, Morrissette et al. [3] evaluated the primary factors governing annual variation in productivity of greater snow geese. Climatic variables in the Arctic such as the spring North Atlantic Oscillation index, snow depth upon arrival at the breeding site and, to a lesser extent, mean temperature during the brood-rearing period in summer have the most impact on the proportion of juveniles in the autumn population. Predation pressure, which can be inferred by lemming abundance (in years of high lemming abundance, predation on goose eggs, an alternative prey, is low, and vice versa), also explained a significant amount of variation in goose productivity. We thus obtained these data for our study years (2007–2009) using the same sources as Morrissette et al. [3].
(g). Statistical analysis
We first used repeated ANOVAs to test the effect of: (i) our treatment type (TRT), which included two levels (fed and fasting), (ii) treatment duration (2, 3 or 4 DAYS), and (iii) year (YEAR) on body mass. Second, we used logistic regressions to test the effect of: (i) TRT (control, fed and fasting), (ii) DAYS (0, 2, 3 or 4 days), (iii) YEAR, and (iv) body mass (either at capture: Mass, or after treatment: Mass2). We also tested for a potential nonlinear relationship between reproductive success and body mass by including a quadratic effect (Mass2), and (v) the difference between mass of individuals at capture and at release (ΔMass, i.e. mass loss in captivity) on the probability of resighting in autumn and on reproductive success (0, resighted without young and 1, resighted with young). All covariates were standardized prior analyses. For the null model (intercept only), the over-dispersion parameter (ĉ = 1.002) indicated that our model fitted the data well. We also included all possible two-way interactions between covariates and years. When an interaction (×) was used, the corresponding fixed effects were also incorporated in the model. Models combining various factors were ranked based on Akaike's Information Criterion (AICc) corrected for small sample size [30] to find the most parsimonious model. We used glm, lme (for repeated ANOVAs where individual was set as a random factor) function and AICc modavg package in R v. 2.11 (R Development Core Team 2010). All means are presented with s.e. throughout.
3. Results
(a). Proximate effects of treatments
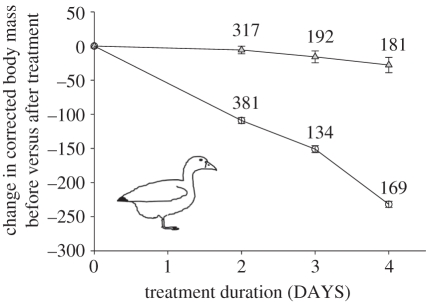
Body mass was affected by the food treatment, captivity duration and year (electronic supplementary material, table S1). Body mass decreased markedly in the fasting groups (daily corrected mass loss = −66.0 g d−1 ± 8.73), but remained fairly stable in the fed groups (daily corrected mass loss = −9.56 g d−1 ± 8.79; figure 1). Body masses at capture also varied among years (electronic supplementary material, table S1). In 2008, body mass at capture was greater (mean mass = 2815.1 ± 8.6 g) than in 2007 (2737.1 ± 8.6 g) and 2009 (2731.9 ± 8.3 g). A significant interaction between year and treatment was also found (electronic supplementary material, table S1). Body mass in the fed groups decreased in 2008 (mean ΔMass = −48.8 ± 8.5 g), while it increased slightly in 2007 (mean ΔMass = 22. 3 ± 8.3 g) and remained relatively stable in 2009 (mean ΔMass = −15.4 ± 6.9 g). Pre- and post-treatment body mass were highly correlated (for each year, all adjusted r2 > 0.59; all F > 700; all p < 0.001).
Figure 1.
Mean change in body mass (body mass controlled for body size and date in grams) before versus immediately after treatment for fed (grey triangles) or fasting (open circles) groups maintained in captivity for 2, 3 or 4 days. Error bars represent ±1 s.e.
(b). Annual breeding conditions
Breeding conditions for geese in the Arctic were highly favourable in 2008 as the North Atlantic Oscillation index was negative (which indicates warm temperatures and an early spring in the eastern Canadian Arctic; [3]), snow depth at arrival was very low, summer temperatures were moderately warm and lemmings were very abundant (table 2). The opposite, unfavourable conditions prevailed in spring 2009 (except for summer temperature), whereas intermediate values occurred in 2007. The proportion of juveniles recorded during the autumn confirmed that 2008 was a highly favourable year for goose reproduction, whereas 2009 was unfavourable and 2007 near average.
Table 2.
Values of the main environmental factors known to affect annual productivity in greater snow geese (from Morrissette et al. [3]) and productivity measured in autumn during the 3 years of the study.
| NAO springa | spring snow depth (cm)b | mean temperature mid-summer (°C)c | lemming abundance (no. lemmings per ha)d | inferred breeding conditione | autumn productivity (%)f | |
|---|---|---|---|---|---|---|
| 2007 | 0.66 | 12.65 ± 1.10 | 6.66 ± 0.26 | 0.22 ± 0.10 | average | 20.6 |
| 2008 | −1.73 | 4.41 ± 1.02 | 6.52 ± 0.34 | 3.49 ± 0.46 | highly favourable | 40.0 |
| 2009 | 1.68 | 30.60 ± 1.58 | 8.92 ± 0.41 | 0.09 ± n.a. | unfavourable | 11.0 |
aNorth Atlantic Oscillation index in May, (data from http://www.cpc.ncep.noaa.gov).bData from Bylot Island (G. Gauthier 2007–2009, unpublished data). Snow depth was measured every 2 days at 50 stations along two 250 m transects. Mean daily snow depth (1–13 June) was used as a snow condition index.cMean summer temperature was calculated from 15 July to 16 August.dBrown lemming monitored annually on Bylot Island using live-trapping (see Gruyer et al. [31] for methods).eBased on the model of Morrissette et al. [3].fProportion of juvenile in the autumn flocks estimated during repeated ground counts along the St Lawrence estuary (see Morrissette et al. [3] for methods; J. Lefebvre 2007–2009, unpublished data, Canadian Wildlife Service). Mean long-term autumn productivity = 24.2%.
(c). Ultimate effect of treatment
We first tested the probability that a female marked in spring was resighted in the subsequent autumn. Resighting probability was only influenced by year with a greater resighting probability in 2009 compared with 2007 and 2008 (χ2 = 62.1, p < 0.001) owing to a higher resighting effort that year. Treatment, treatment duration or body mass at capture did not significantly influence resighting probability (all χ2 < 4.5, all p > 0.10). This suggests that treatment effects on reproductive success (see below) are not biased by variable resighting probabilities among treated groups.
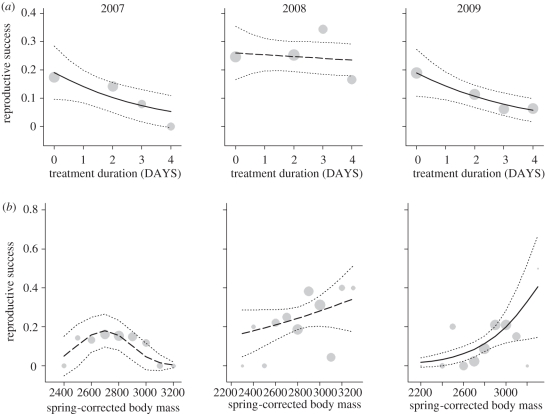
Initial or post-treatment body mass had similar effects on reproductive success, but models with initial body mass were slightly better ranked (electronic supplementary material, table S2). The following interpretation is thus based on the latter analysis. Seven models explaining reproductive success were equivalent on the basis of ΔAICc (i.e. ΔAICc < 2; electronic supplementary material, table S2). All these models retained year in interaction with body mass or body mass2 and treatment duration (cumulative AICc weight = 0.84). The type of treatment (fed or fasting) and mass difference before and after treatment (ΔMass), however, were not included in the most parsimonious models (electronic supplementary material, table S2). To examine the interactions with years, we built separate models for each year. In 2007 and 2009, the preferred models retained treatment duration and body mass, and in 2007, body mass2 as well, but no interactions. However, in 2008, the null model was retained (ΔAICc of 4.04 compared with a model with treatment and body mass effects) and treatment duration did not affect reproductive success that year (table 3 and figure 2a). In both 2007 and 2009, treatment duration (DAYS) was negatively related to reproductive success (table 3 and figure 2a). During these 2 years, breeding success decreased on average by 45, 60 and 71 per cent after 2, 3 and 4 days of captivity respectively (figure 2a). In addition, body mass showed a significant positive relationship with reproductive success in 2009 and a non-significant positive trend in 2008. Interestingly, reproductive success decreased in birds of both relatively low and high body mass in 2007 (table 3 and figure 2b).
Table 3.
Estimates of the three most important parameters explaining reproductive success (probability that a female was resighted in the subsequent autumn with at least one young; 0, resighted without young and 1, resighted with young) of greater snow geese (according to model selection in electronic supplementary material, table S2) for each year (2007–2009) analysed separately. (DAYS, number of days in captivity (0, 2, 3 or 4 days); Mass, standardized body mass at capture controlled for body size and date (see text).)
| year | covariate | estimate | s.e. | p-value |
|---|---|---|---|---|
| 2007 | intercept | −1.80 | 0.41 | <0.01 |
| DAYS | −0.37 | 0.18 | 0.04 | |
| Mass | 20.49 | 12.82 | 0.11 | |
| Mass2 | −20.62 | 12.79 | 0.11 | |
| 2008 | intercept | −1.07 | 0.27 | <0.01 |
| DAYS | −0.03 | 0.11 | 0.78 | |
| Mass | 0.23 | 0.17 | 0.17 | |
| Mass2 | −0.03 | 0.11 | 0.78 | |
| 2009 | intercept | −1.37 | 0.28 | <0.01 |
| DAYS | −0.38 | 0.13 | <0.01 | |
| Mass | 0.51 | 0.21 | 0.02 | |
| Mass2 | −3.07 | 5.06 | 0.54 |
Figure 2.
Mean reproductive success (probability that a female was resighted in the subsequent autumn with at least one young) in relation to (a) the treatment duration (days maintained in captivity) or (b) with body mass in spring (body mass at capture controlled for body size and date), in 2007–2009. The fitted logistic models (black line when significant and long dashed otherwise) as well as its confidence interval at 95% (stippled line) are shown. Grey circle sizes are proportional to log (N) available for each days of treatment or body mass classes.
4. Discussion
Our results experimentally demonstrate that events occurring during spring can carry-over to affect the reproductive output of long-distance, arctic-nesting migrants. To our knowledge, this study is the first experiment examining reproductive consequences of COEs at the individual level. In their recent review, Harrison et al. [4] propose an experimental framework to estimate the relative contribution of extrinsic (environmental conditions) and intrinsic (individual characteristics) quality effects. Our experimental design (manipulation of individual state in multiple years) embraced this framework, and our data indicate that the absence of COEs in one year (2008) was owing to extrinsic factors. Indeed, reproductive success at the population level was exceptionally good in 2008 owing to highly favourable environmental conditions at the Arctic breeding site. It thus appears that these good breeding conditions were sufficient to offset the COE of our manipulation, which was not the case under average or poor breeding conditions.
We also examined the mechanisms through which COEs could be mediated, through changes in body condition (body stores) or through other processes, such as acute stress induced by the manipulation. Our analysis of reproductive success provides support for mechanisms other than a punctual decrease in body condition. Indeed, in 2 out of 3 years, control groups had greater breeding success than all treated birds, regardless of the feeding treatment. Even though the feeding treatment clearly impacted the body mass of geese (after 4 days of treatment, body mass difference between fasting and fed groups reached 184 g, i.e. 6.6% of total body mass), it had no apparent repercussions on reproductive success. Moreover, the reduction in reproductive success was proportional to the duration of the captivity, which supports the captivity-induced stress hypothesis. Nonetheless, it is still possible that physiological changes induced by the stress of captivity subsequently resulted in a lower body condition in manipulated females (see below). An alternative interpretation could be that time spent in captivity delayed departure from the main spring staging site, leading to a delayed arrival in the Arctic and laying, which reduced reproductive success [13,32]. Although we do not know if departure was delayed by our treatment, it is an unlikely mechanism explaining the observed COEs in our study system for at least two reasons. First, our experimental manipulation was not conducted during the goose departure period and took place one to two weeks before massive departure from the staging site. Second, Bêty et al. [13] showed that individual departure dates from the main staging area are well synchronized in this population and are not related to departure body condition, arrival date or laying date in the Arctic.
Our measure of reproductive success is the probability of being resighted with at least one young in autumn, and not the total number of young produced. We did not find any effect of body mass reduction in our experimental treatments on the former index of reproductive success, but it may still have induced more subtle effects that we could not detect. Bêty et al. [13] suggested a link between spring body condition, laying date and clutch size in greater snow geese. Thus, it is still possible that our manipulation of condition could have had an effect on the number of young produced, which we could not investigate in our study. However, a recent experimental reduction in body condition during the pre-laying period in the common eider (Somateria mollissima) confirmed a relationship between condition and laying date, but not in clutch size reduction [33].
Even though fed females more or less maintained their body mass during captivity, they did not gain mass as wild birds would have done (based on the average rate of body mass gain in spring, females should have gained 51 g over a 4 day period; see §3). Acute stress experienced during captivity probably explains why geese did not fully exploit the experimental food resources. Captivity is known to reduce body mass and foraging success [34,35] and the stress experienced during captivity may even have persisted for some time after release [36]. In birds, chronic stress can reduce body condition [37,38] and thus, it is probable that birds stressed by captivity eventually ended their spring staging period with reduced body condition. If the elevated corticosterone levels associated with the acute stress of capture persisted for some time [39], it could also have deleterious effects on the reproductive physiology and behaviour [40,41]. For instance, Goutte et al. [41] found that elevated levels of baseline corticosterone during the pre-laying period were associated with a higher probability of skipping breeding in female snow petrel (Pagodroma nivea). Our experimental design also manipulated males because they were maintained in captivity for the same length of time as females (though all of them received ad libitum food) to ensure that mates could reunite upon release. Even if males do not incur the costs of egg-laying and incubation, they do invest in reproduction and also rely on endogenous reserves [42]. Males in poor condition may be less vigilant and thus less efficient at protecting their mate or the nest [25]. Therefore, we cannot rule out the possibility that some of the COEs that we documented on reproduction may have been amplified through a negative effect of our manipulation of male condition.
Earlier studies have shown that body condition in spring is a key factor driving the reproductive outputs of arctic-nesting geese [16,19–22]. In the present study, individual body condition was significantly related to reproductive success only in 2009, the year when environmental conditions in the Arctic were the least favourable, although a similar trend was observed in 2008. Interestingly, the mean body condition of birds was highest in 2008, the year where productivity of the population was highest. In contrast to some other goose species, greater snow geese use a mixed capital/income breeding strategy as a large amount of nutrients invested in the eggs are acquired in the Arctic [43]. It is thus possible that in 2009, birds had to rely more extensively on body reserves acquired on the main staging site to breed successfully than in other years owing to the unfavourable environmental conditions that prevailed in the Arctic that year. The quadratic effect found in 2007 suggests that an optimal departure body mass for migration could also exist and may vary depending on environmental conditions encountered en route or on the breeding grounds. This potentially illustrates a reproductive cost of being too fat for migrants, such as higher metabolic demands under some conditions [44]. Moreover, in our study population, females in better condition at departure initiate laying earlier than those in lower condition [13]. Although early breeding birds typically reach higher reproductive success, some component of the reproductive success can be significantly depressed for the earliest breeders in some years [32]. Further investigations are needed to tease apart the alternative hypotheses.
This study is to our knowledge, the first experimental manipulation showing that events occurring on distant staging areas can have a strong influence on reproductive success. Until now, evidence for such COEs solely came from correlative studies [3,4,11,12]. Our results also suggest that conditions experienced on the breeding grounds can enhance or buffer COEs. Despite our elaborate experimental design, elucidating the mechanisms involved proved more difficult. Direct change in body stores induced by our manipulation apparently had no effect on reproductive success. Stress induced by our manipulation was probably responsible (directly or indirectly) for the observed COE, although a delay in migration phenology could also be involved. Other studies have emphasized the need to explore the role of connectivity between stopover and breeding sites [4,16,45] but we argue that significant progresses in this field will require more experimental manipulations [4]. These experiments should aim to elucidate the mechanisms involved and to explore how environmental conditions encountered during late migration and breeding can modulate the strength of COEs.
Acknowledgements
This experiment was approved by the Committee of Animal Protection of the Université du Québec à Rimouski (Authorization number: CPA-42-10-78).
This study was funded by the Natural Science and Engineering Research Council of Canada (NSERC), the Fonds Québécois de Recherche sur la Nature et les Technologies (FQRNT) and the Canadian Wildlife Service. P.L. was supported by a fellowship from the FQRNT, and P.L.F.F. was supported by NSERC, the W. Garfield Weston Award for Northern Research and the Canadian Wildlife Federation. We are grateful to all fieldworkers especially Gérald Picard, Johanne Dussureault, Marie-Claude Martin and Eliane Valiquette. We thank Marie-Christine Cadieux for managing the databases. We thank Frédéric Angelier, Peter Marra, Guillaume Souchay and two anonymous reviewers for helpful comments on the manuscript.
References
- 1.Newton I. 2006. Can conditions experienced during migration limit the population levels of birds? J. Ornithol. 147, 146–166 10.1007/s10336-006-0058-4 (doi:10.1007/s10336-006-0058-4) [DOI] [Google Scholar]
- 2.Fryxell J. M., Sinclair A. R. E. 1988. Causes and consequences of migration by large herbivores. Trends Ecol. Evol. 3, 237–241 10.1016/0169-5347(88)90166-8 (doi:10.1016/0169-5347(88)90166-8) [DOI] [PubMed] [Google Scholar]
- 3.Morrissette M., Bêty J., Gauthier G., Reed A., Lefebvre J. 2010. Climate, trophic interactions, density dependence and carry-over effects on the population productivity of a migratory Arctic herbivorous bird. Oikos 119, 1181–1191 10.1111/j.1600-0706.2009.18079.x (doi:10.1111/j.1600-0706.2009.18079.x) [DOI] [Google Scholar]
- 4.Harrison X. A., Blount J. D., Inger R., Norris D. R., Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18 10.1111/j.1365-2656.2010.01740.x (doi:10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 5.Newton I. 1998. Population limitation in birds. New York, NY: Academic Press [Google Scholar]
- 6.Norris D. R., Taylor C. M. 2006. Predicting the consequences of carry-over effects for migratory populations. Biol. Lett. 2, 148–151 10.1098/rsbl.2005.0397 (doi:10.1098/rsbl.2005.0397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Runge M., Marra P. P. 2005. Modeling seasonal interactions in the annual cycle of migratory birds. In Birds of two worlds: the ecology and evolution of temperate-tropical migration systems (eds Greenberg R., Marra P. P.), pp. 375–389 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 8.Cresswell W. 2008. Non-lethal effects of predation in birds. Ibis 150, 3–17 10.1111/j.1474-919X.2007.00793.x (doi:10.1111/j.1474-919X.2007.00793.x) [DOI] [Google Scholar]
- 9.Visser M. E., Perdeck A. C., Van Balen J. H., Both C. 2009. Climate change leads to decreasing bird migration distances. Global Change Biol. 15, 1859–1865 10.1111/j.1365-2486.2009.01865.x (doi:10.1111/j.1365-2486.2009.01865.x) [DOI] [Google Scholar]
- 10.Wingfield J. C., Ramenofsky M. 1999. Hormones and the behavioral ecology of stress. In Stress physiology in animals (ed. Baum P. H. M.), pp. 1–51 Sheffield, UK: Sheffield Academic Press [Google Scholar]
- 11.Marra P. P., Hobson K. A., Holmes R. T. 1998. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282, 1884–1886 10.1126/science.282.5395.1884 (doi:10.1126/science.282.5395.1884) [DOI] [PubMed] [Google Scholar]
- 12.Saino N., Szep T., Romano M., Rubolini D., Spina F., Moller A. P. 2004. Ecological conditions during winter predict arrival date at the breeding quarters in a trans-Saharan migratory bird. Ecol. Lett. 7, 21–25 10.1046/j.1461-0248.2003.00553.x (doi:10.1046/j.1461-0248.2003.00553.x) [DOI] [Google Scholar]
- 13.Bêty J., Gauthier G., Giroux J. F. 2003. Body condition, migration, and timing of reproduction in snow geese: a test of the condition-dependent model of optimal clutch size. Am. Nat. 162, 110–121 [DOI] [PubMed] [Google Scholar]
- 14.Norris D. R., Marra P. P., Montgomerie R., Kyser T. K., Ratcliffe L. M. 2004. Reproductive effort molting latitude, and feather color in a migratory songbird. Science 306, 2249–2250 10.1126/science.1103542 (doi:10.1126/science.1103542) [DOI] [PubMed] [Google Scholar]
- 15.Studds C. E., Marra P. P. 2005. Nonbreeding habitat occupancy and population processes: an upgrade experiment with a migratory bird. Ecology 86, 2380–2385 10.1890/04-1145 (doi:10.1890/04-1145) [DOI] [Google Scholar]
- 16.Drent R. H., Eichhorn G., Flagstad A., Van der Graaf A. J., Litvin K. E., Stahl J. 2007. Migratory connectivity in Arctic geese: spring stopovers are the weak links in meeting targets for breeding. J. Ornithol. 148, S501–S514 10.1007/s10336-007-0223-4 (doi:10.1007/s10336-007-0223-4) [DOI] [Google Scholar]
- 17.Ebbinge B. S., Spaans B. 1995. The importance of body reserves accumulated in spring staging areas in the temperate zone for breeding in dark-bellied brent geese Branta bernicla in the high Arctic. J. Avian Biol. 26, 105–113 10.2307/3677058 (doi:10.2307/3677058) [DOI] [Google Scholar]
- 18.Alisauskas R. T. 2002. Arctic climate, spring nutrition, and recruitment in midcontinent lesser snow geese. J. Wildl. Manag. 66, 181–193 10.2307/3802884 (doi:10.2307/3802884) [DOI] [Google Scholar]
- 19.Klaassen M., Bauer S., Madsen J., Ingunn T. 2006. Modelling behavioural and fitness consequences of disturbance for geese along their spring flyway. J. Appl. Ecol. 43, 92–100 10.1111/j.1365-2664.2005.01109.x (doi:10.1111/j.1365-2664.2005.01109.x) [DOI] [Google Scholar]
- 20.Bechet A., Giroux J. F., Gauthier G. 2004. The effects of disturbance on behaviour, habitat use and energy of spring staging snow geese. J. Appl. Ecol. 41, 689–700 10.1111/j.0021-8901.2004.00928.x (doi:10.1111/j.0021-8901.2004.00928.x) [DOI] [Google Scholar]
- 21.Mainguy J., Bety J., Gauthier G., Giroux J. F. 2002. Are body condition and reproductive effort of laying greater snow geese affected by the spring hunt? Condor 104, 156–161 10.1650/0010-5422(2002)104[0156:ABCARE]2.0.CO;2 (doi:10.1650/0010-5422(2002)104[0156: ABCARE]2.0.CO;2) [DOI] [Google Scholar]
- 22.Gauthier G., Giroux J. F., Reed A., Bechet A., Belanger L. 2005. Interactions between land use, habitat use, and population increase in greater snow geese: what are the consequences for natural wetlands? Global Change Biol. 11, 856–868 10.1111/j.1365-2486.2005.00944.x (doi:10.1111/j.1365-2486.2005.00944.x) [DOI] [Google Scholar]
- 23.Gauthier G., Giroux J. F., Bedard J. 1992. Dynamics of fat and protein reserves during winter and spring migration in greater snow geese. Can. J. Zool.-Rev. Can. Zool. 70, 2077–2087 10.1139/z92-280 (doi:10.1139/z92-280) [DOI] [Google Scholar]
- 24.Prevett J. P., Macinnes C. D. 1980. Family and other social groups in snow geese. Wildl Monogr. 71, 1–46 [Google Scholar]
- 25.Gauthier G., Tardif J. 1991. Female feeding and male vigilance during nesting in greater snow geese. Condor 93, 701–711 10.2307/1368202 (doi:10.2307/1368202) [DOI] [Google Scholar]
- 26.Gauthier G., Pradel R., Menu S., Lebreton J. D. 2001. Seasonal survival of greater snow geese and effect of hunting under dependence in sighting probability. Ecology 82, 3105–3119 10.1890/0012-9658(2001)082[3105:SSOGSG]2.0.CO;2 (doi:10.1890/0012-9658(2001) 082[3105:SSOGSG]2.0.CO;2) [DOI] [Google Scholar]
- 27.Feret M., Bety J., Gauthier G., Giroux J. F., Picard G. 2005. Are abdominal profiles useful to assess body condition of spring staging greater snow geese? Condor 107, 694–702 10.1650/0010-5422(2005)107[0694:AAPUTA]2.0.CO;2 (doi:10.1650/0010-5422(2005)107 [0694:AAPUTA]2.0.CO;2) [DOI] [Google Scholar]
- 28.Menu S., Gauthier G., Reed A. 2005. Survival of young greater snow geese (Chen caerulescens atlantica) during fall migration. Auk 122, 479–496 10.1642/0004-8038(2005)122[0479:SOYGSG]2.0.CO;2 (doi:10.1642/0004-8038(2005)122[0479:SOYGSG]2.0.CO;2) [DOI] [Google Scholar]
- 29.Reed E. T. 2003. Coûts des soins parentaux et effet des conditions environnementales sur la reproduction de la Grande Oie des neiges. PhD dissertation, Laval University, Québec, Canada [Google Scholar]
- 30.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference, 2nd edn New York, NY: Springer [Google Scholar]
- 31.Gruyer N., Gauthier G., Berteaux D. 2010. Demography of two lemming species on Bylot Island, Nunavut, Canada. Polar Biol. 33, 725–736 10.1007/s00300-009-0746-7 (doi:10.1007/s00300-009-0746-7) [DOI] [Google Scholar]
- 32.Lepage D., Gauthier G., Menu S. 2000. Reproductive consequences of egg-laying decisions in snow geese. J. Anim. Ecol. 69, 414–427 10.1046/j.1365-2656.2000.00404.x (doi:10.1046/j.1365-2656.2000.00404.x) [DOI] [Google Scholar]
- 33.Descamps S., Bêty J., Love O., Gilchrist G. 2011. Individual optimization of reproduction in a long-lived migratory bird: a test of the condition-dependent model of laying date and clutch size. Funct. Ecol. 25, 671–681 10.1111/j.1365-2435.2010.01824.x (doi:10.1111/j.1365-2435.2010.01824.x) [DOI] [Google Scholar]
- 34.Ekman J. B., Hake M. K. 1990. Monitoring starvation risk: adjustments of body reserves in greenfinches (Carduelis chloris L.) during periods of unpredictable foraging success. Behav. Ecol. 1, 62–67 10.1093/beheco/1.1.62 (doi:10.1093/beheco/1.1.62) [DOI] [Google Scholar]
- 35.Butlers S. J., Whittingham M. J., Quinn J. L., Cresswell W. 2006. Time in captivity, individual differences and foraging behaviour in wild-caught chaffinches. Behaviour 143, 535–548 10.1163/156853906776240632 (doi:10.1163/156853906776240632) [DOI] [Google Scholar]
- 36.Dickens M. J., Delehanty D. J., Romero L. M. 2009. Stress and translocation: alterations in the stress physiology of translocated birds. Proc. R. Soc. B 276, 2051–2056 10.1098/rspb.2008.1778 (doi:10.1098/rspb.2008.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busch D. S., Sperry T. S., Peterson E., Do C. T., Wingfield J. C., Boyd E. H. 2008. Impacts of frequent, acute pulses of corticosterone on condition and behavior of Gambel's white-crowned sparrow (Zonotrichia leucophtys gambelii). Gen. Comp. Endocrinol. 158, 224–233 10.1016/j.ygcen.2008.07.010 (doi:10.1016/j.ygcen.2008.07.010) [DOI] [PubMed] [Google Scholar]
- 38.Rich E. L., Romero L. M. 2005. Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R1628–R1636 10.1152/ajpregu.00484.2004 (doi:10.1152/ajpregu.00484.2004) [DOI] [PubMed] [Google Scholar]
- 39.Legagneux P., Gauthier G., Chastel O., Picard G., Bêty J. 2011. Do fecal samples from captured birds in the wild reveal baseline corticosterone? A case study in the greater snow geese (Chen caerulescens). Gen. Comp. Endocrinol. 172, 440–445 10.1016/j.ygcen.2011.04.009 (doi:10.1016/j.ygcen.2011.04.009) [DOI] [PubMed] [Google Scholar]
- 40.Schoech S. J., Rensel M. A., Bridge E. S., Boughton R. K., Wilcoxen T. E. 2009. Environment, glucocorticoids, and the timing of reproduction. Gen. Comp. Endocrinol. 163, 201–207 10.1016/j.ygcen.2008.09.009 (doi:10.1016/j.ygcen.2008.09.009) [DOI] [PubMed] [Google Scholar]
- 41.Goutte A., Antoine E., Weimerskirch H., Chastel O. 2010. Age and the timing of breeding in a long-lived bird: a role for stress hormones? Funct. Ecol. 24, 1007–1016 10.1111/j.1365-2435.2010.01712.x (doi:10.1111/j.1365-2435.2010.01712.x) [DOI] [Google Scholar]
- 42.Choinière L., Gauthier G. 1995. Energetics of reproduction in female and male greater snow geese. Oecologia 103, 379–389 10.1007/BF00328628 (doi:10.1007/BF00328628) [DOI] [PubMed] [Google Scholar]
- 43.Gauthier G., Bety J., Hobson K. A. 2003. Are greater snow geese capital breeders? New evidence from a stable-isotope model. Ecology 84, 3250–3264 10.1890/02-0613 (doi:10.1890/02-0613) [DOI] [Google Scholar]
- 44.Witter M. S., Cuthill I. C. 1993. The ecological costs of avian fat storage. Phil. Trans. R. Soc. B 340, 73–92 10.1098/rstb.1993.0050 (doi:10.1098/rstb.1993.0050) [DOI] [PubMed] [Google Scholar]
- 45.Webster M. S., Marra P. P., Haig S. M., Bensch S., Holmes R. T. 2002. Links between worlds: unraveling migratory connectivity. Trends Ecol. Evol. 17, 76–83 10.1016/S0169-5347(01)02380-1 (doi:10.1016/S0169-5347(01)02380-1) [DOI] [Google Scholar]