Abstract
How do biogeographically different provinces arise in response to oceanic barriers to dispersal? Here, we analyse how traits related to the pelagic dispersal and adult biology of 985 tropical reef fish species correlate with their establishing populations on both sides of two Atlantic marine barriers: the Mid-Atlantic Barrier (MAB) and the Amazon–Orinoco Plume (AOP). Generalized linear mixed-effects models indicate that predictors for successful barrier crossing are the ability to raft with flotsam for the deep-water MAB, non-reef habitat usage for the freshwater and sediment-rich AOP, and large adult-size and large latitudinal-range for both barriers. Variation in larval-development mode, often thought to be broadly related to larval-dispersal potential, is not a significant predictor in either case. Many more species of greater taxonomic diversity cross the AOP than the MAB. Rafters readily cross both barriers but represent a much smaller proportion of AOP crossers than MAB crossers. Successful establishment after crossing both barriers may be facilitated by broad environmental tolerance associated with large body size and wide latitudinal-range. These results highlight the need to look beyond larval-dispersal potential and assess adult-biology traits when assessing determinants of successful movements across marine barriers.
Keywords: macroecology, biogeographic barriers, Amazon–Orinoco Plume, rafting, larval-development mode, body size
1. Introduction
Geographical barriers are important determinants of the evolution and distributions of animals and plants. For marine organisms, barriers to dispersal are often subtle and present a challenge for evolutionary ecologists seeking to understand population structure and speciation in the sea, and the formation of biogeographic provinces with distinctive biotas [1–3]. Aside from obvious physical obstacles such as landmasses, permeable or ‘soft’ aquatic barriers limit the distributions of marine organisms. Examples of such barriers include both large stretches of deep oceanic water [4–6] and near shore gradients in physical and chemical properties of sea water [2,7], both of which reduce the potential for ocean-wide colonization by near shore organisms. Some species have physiological, morphological, ecological and/or behavioural traits that improve their likelihood of overcoming such obstacles [6,8]. Thus, these barriers act as dispersal ‘filters’ that impact selected species rather than absolute barriers that are impassable to all [4,9].
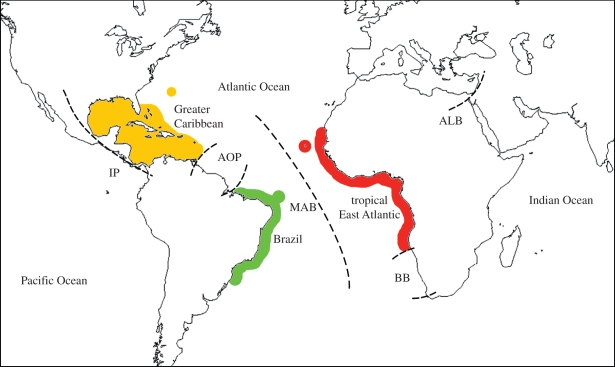
Five biogeographic barriers have shaped the large-scale distributions of the present-day reef fish fauna of the tropical Atlantic Ocean: the Central American Land Bridge, the Mid-Atlantic Barrier (MAB; the stretch of tropical ocean between equatorial America and Africa), the Amazon–Orinoco Plume (AOP) along the northeast coast of South America, the cold Benguela upwelling zone off southwest Africa and the Arabian Land Bridge between Africa and Asia [3,10,11] (figure 1). The Isthmus of Panama, the Benguela Barrier and the Arabian Land Bridge isolate the Atlantic Ocean from other ocean basins, whereas the MAB and the AOP are major determinants of regional endemism patterns within the tropical Atlantic Ocean [2,3,5]. Here, we focus on the MAB and the AOP because (i) both are permeable, allowing some fish species to expand their geographical ranges over them, (ii) they allow investigation of potentially contrasting effects of two quite different types of barriers on a single Atlantic fauna of tropical reef fishes, and (iii) data on reef fish species distributions in relation to these two barriers are readily available [3]. The ongoing permeability of the MAB and the AOP is evidenced by genetic analysis of established populations from both sides of the barriers [12–16] and by observations of ‘vagrants’ that successfully crossed the barriers but have not established populations in the new area [17]. The MAB and the AOP differ in their time of existence and mode of operation. The former is a deep-ocean barrier that was produced by the formation of the Atlantic Ocean basin as Africa and South America separated over the past 85 Myr. This gradually created a gap that has expanded to as much as approximately 3500 km across the equatorial Atlantic zone, where major east–west currents flow (although with a minimum straight-line distance between the continents of approx. 2800 km). This represents an extreme distance relative to regular larval dispersal by marine organisms [3]. By contrast, the approximately 10 Myr-old AOP is a coastal barrier formed by the formidable freshwater and sediment discharges of the Amazon and Orinoco rivers spreading along 2300 km of the northeast coast of South America [2]. These outflows produce dramatic changes in the physical and chemical properties of coastal waters in the area [2,18]. In the AOP, a thick (approx. 30 m deep) turbid, low-salinity layer at the surface effectively reduces connectivity of northern and southern populations of many coastal marine organisms [2]. Unlike the oceanic MAB, which only allows pelagic dispersal, the AOP is an inshore barrier which contains benthic habitat that potentially is available to reef fish species that can use non-reef habitats and tolerate reduced salinities.
Figure 1.
Biogeographic provinces and barriers affecting tropical reef fishes in the Atlantic Ocean. Land barriers: IP, Isthmus of Panama; ALB Arabian Land Bridge. Soft barriers: AOP, Amazon–Orinoco Plume; MAB, Mid-Atlantic Barrier; BB, Benguela Barrier.
The effectiveness of permeable barriers such as the AOP and the MAB on range expansion and regional faunal composition may be influenced by different species-level traits that affect not only the pelagic dispersal but also the potential to establish a new population after a barrier has been crossed. Variation in the larval-development mode of tropical reef fishes has been proposed as a determinant of species geographical ranges through its effects on the duration of the pelagic larval period [19–22]. Eggs from fishes that spawn in the water column (pelagic spawners) are subjected to ocean currents immediately after spawning, whereas eggs from demersal spawners are attached to the bottom and the larvae can disperse only after hatching, several days after spawning. It is generally assumed that the dispersal potential of larvae of pelagic spawners is greater than that of demersal spawners because propagules of the former spend more time in the pelagic stage [22].
Pelagic-dispersal potential is also influenced by the ability of species to raft with floating debris in the open sea. Marine organisms that raft as juveniles or adults are capable of crossing large expanses of ocean [23–25], and rafting by tropical reef fishes may be more common than previously thought [6,14,26]. Rafting may be an important dispersal mechanism because it facilitates the dispersal of multiple life stages (e.g. juveniles to adults) and is independent of the duration of the pelagic larval phase. Currently, however, we lack a general understanding of the significance of rafting as a mechanism for traversing large dispersal barriers by tropical reef fishes [6,26].
Expansion of a species geographical range to the far side of a barrier must be affected by its capacity to establish a population in the new habitat following dispersal across the barrier, which depends on the ability of adults and the larvae they produce to exploit new ecological conditions. The degree of adult and larval tolerance for a range of environmental conditions could thus affect the success of establishment. Among both terrestrial and marine organisms, large-bodied species tend to have broader geographical distributions [27,28], and large size may facilitate establishment by providing a degree of eurytolerance [9,29]. As indicators of tolerance for a range of environmental conditions by crossers of both the MAB and the AOP, we used adult body size, latitudinal-range within a province, and adult usage of both reef and non-reef habitats. Other adult traits we examined relate more to the potential for adults to live in habitat of the coastal AOP: use of brackish habitats and the ability to live in depths below the freshwater plume. These two traits, plus use of non-reef habitats, could determine which species can use saline non-reef (sponge) habitats under the floating freshwater plume of the AOP [18], or in brackish, non-reef habitats within the plume itself.
In this study, we use a comprehensive compilation of species-level larval and adult traits described above and the geographical distributions of tropical reef fish species to investigate which traits predict range expansion across the MAB and the AOP. We then discuss how those traits relate to the distinctive mode of operation of each barrier. Our existing database on large-scale distributions of members of this fauna [3] greatly facilitated this analysis.
2. Material and methods
(a). Data collection
Existing data on larval-development mode, association with marine flotsam, geographical distributions on each side of each barrier, latitudinal-range, maximum total length (our surrogate for body size) and maximum depth of the depth range were collected for 985 reef-associated tropical Atlantic fish species (electronic supplementary material, appendix S1). These included demersal and semi-pelagic species that typically associate with coral, rocky and/or coralline algal reefs. For Western-Atlantic species, data on reef species use of non-reef habitats (sand, mud, mangroves, seagrasses and other submerged vegetation, sponge beds), brackish habitat use and latitudinal-range within a province (the Greater Caribbean or Brazil) were taken from the literature, online databases (www.fishbase.org) and complemented from our own records.
Distributions relating to the MAB and the AOP came from a large database previously used by Floeter et al. (appendix S1 of [3]). Occurrence records include both established species and rare waifs, which could represent either rare arrivals or the last survivors at the end of a failed establishment following arrival in abundance. Occurrence records thus include information about both crossings and establishment, although the great majority (95.3%) of trans-barrier species are successful crossers which have established populations on both sides of a barrier.
Knowledge on the latitudinal-ranges and habitat use of species restricted to the tropical East Atlantic is insufficient for inclusion in analyses involving those two variables, which are limited to species resident in the West Atlantic. Latitudinal-range data came from regional databases (www.fishbase.org) [30] and the collection record database provided by the global aggregator Ocean and Biogeographic Information System (www.iobis.org). We calculated the intra-regional latitudinal-range of each species within either the Greater Caribbean or Brazilian provinces and used the larger of the two values for species found in both. Maximum total length data were mostly obtained from the literature [30–34]. Where length data could not be found (only 1.9% of the species), the mean maximum total length for the genus was used instead. Data on use of non-reef habitats, brackish habitats and maximum depth came from the same sources as the length data (and also [35]). Larval-development modes were assigned to each species following the classification by Thresher [19]: pelagic spawners (which release small, rapidly developing planktonic eggs into the water column at spawning), demersal spawners (which guard or brood slowly developing large demersal eggs to hatching, or give birth directly to swimming young) and balistid-type spawners (which guard rapidly developing small eggs to hatching, and mainly include balistids, monocanthids and tetraodontids). Finally, species were designated as rafters if they have been reported in the literature [24,36] or observed by us aggregating around drifting flotsam in the ocean.
(b). Data analysis
To determine the relative importance of different species-level traits associated with crossing the MAB and the AOP, statistical relationships between species traits and their distribution were investigated using generalized linear mixed-effect models (GLMMs). These models included the taxon (genus nested within family) as a random effect, accounting for the non-independence of species owing to shared evolution [37–39]. For the MAB, we conducted two analyses: (i) resident West-Atlantic species (i.e. excluding species with populations in the East Atlantic but not West Atlantic, and occurring in the West Atlantic only as vagrants, if at all) in order to analyse all trait variables and (ii) all species, but excluding latitudinal-range and habitat use, which were not available for many East-Atlantic species. For the AOP, we conducted a single analysis with all West-Atlantic resident species and all factors. Finally, to test for a possible disproportionate influence of super-dispersing families on the whole-fauna relationships, we repeated the above analyses after removing all species of Carangidae and Muraenidae, which contain many large species that either have semi-pelagic habits and associate with flotsam (Carangids), or possess very long-lived pelagic larvae (Muraenids, see [6,32,40]).
GLMMs allow both continuous and categorical predictor variables, and a nonlinear response variable [41]. Such models also account for the non-independence of species owing to shared evolution by making taxa a random variable [38,39,42,43]. This is accomplished by removing variation owing to differences among families or genera from the error term and allowing them to vary randomly around the overall mean [37,44,45]. Other independent variables (fixed effects) can then be examined, and any significant results can then be generalized to the entire fauna. Barrier crossing was considered to have binomial distributions of errors (crossing = 1 and not crossing = 0; these data were examined using a logit link function). The GLMM was run with the function glmmML from the R package glmmML [46,47]. To determine the best predictive model for crossing potential, we compared the full model with nested models in which one of the predictor variables was dropped (using the ‘ANOVA’ function in the R base statistics distribution). If an ANOVA found a dropped variable to have no significant effect on the model, then that variable was removed from the model (table 1a). Interactions (up to two-way) were examined and dropped in the same fashion. Plots of relationships between crossing and significant factors were derived from probability values produced as part of the output of each GLMM.
Table 1.
Summary of generalized linear mixed-effect models statistics for effects of various traits on the ability of species to overcome two oceanographic barriers. (a) The effect of dropping each variable separately from full models (showing both Akaike information criteria (AIC) and χ2-test statistics). (b) The final predictive model (showing estimates, standard error and p-values). (Coefficient in bold indicates that p-value is significant (p < 0.05)).
| Mid-Atlantic Barrier |
Amazon–Orinoco Plume (770) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Western-Atlantic species only (770) |
all species (985) |
||||||||
| (a) variable | d.f. | AIC | Pr (χ2) | d.f. | AIC | Pr (χ2) | d.f. | AIC | Pr (χ2) |
| full model | 11 | 377.3 | — | 8 | 501.1 | — | 11 | 642.8 | — |
| rafting behaviour | 10 | 383.8 | 0.003 | 7 | 565.6 | <0.001 | 10 | 639.1 | 0.616 |
| larval-development mode | 9 | 376.2 | 0.238 | 6 | 499.3 | 0.336 | 9 | 652.8 | <0.001 |
| maximum body size | 10 | 389.8 | <0.001 | 7 | 523.2 | <0.001 | 10 | 644.0 | 0.061 |
| intra-regional latitudinal-range | 10 | 416.6 | <0.001 | n.a. | n.a. | n.a. | 10 | 793.8 | <0.001 |
| multi-habitat use | 10 | 378.9 | 0.055 | n.a. | n.a. | n.a. | 10 | 645.8 | 0.025 |
| maximum depth | 10 | 375.8 | 0.491 | n.a. | n.a. | n.a. | 10 | 640.8 | 0.978 |
| low-salinity affinity | 10 | 377.3 | 0.160 | n.a. | n.a. | n.a. | 10 | 641.6 | 0.379 |
| (b) variable | est. | s.e. | p | est. | s.e. | p | est. | s.e. | p |
| intercept | −10.324 | 1.174 | <0.001 | −6.258 | 0.699 | <0.001 | −7.026 | 0.818 | < 0.001 |
| rafting behaviour | 1.550 | 0.484 | 0.001 | 2.434 | 0.394 | < 0.001 | n.a. | n.a. | n.a. |
| larval-development mode | |||||||||
| pelagic versus demersal | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.044 | 0.340 | 0.908 |
| pelagic versus balistid | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.721 | 0.827 | 0.382 |
| demersal versus balistid | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | −0.676 | 0.856 | 0.429 |
| maximum body size | 2.310 | 0.538 | <0.001 | 2.572 | 0.426 | <0.001 | 0.832 | 0.386 | 0.031 |
| intra-regional latitudinal-range | 0.200 | 0.038 | <0.001 | n.a. | n.a. | n.a. | 0.256 | 0.026 | <0.001 |
| multi-habitat use | −0.741 | 0.368 | 0.065 | n.a. | n.a. | n.a. | 0.577 | 0.230 | 0.012 |
3. Results
Among the entire suite of 985 species used in our analyses, 11.0 per cent from 71 genera and 35 families have trans-Atlantic distributions that span the MAB. By contrast, 39.5 per cent of 770 Western-Atlantic resident species from 175 genera and 51 families are distributed across the AOP in both Caribbean and Brazilian provinces. Among the Western-Atlantic residents, 87.5 per cent of the 104 species that cross the MAB also cross the AOP. Thus, relative to the MAB, the AOP is crossed by many more species of a much broader range of taxa and ecotypes, and Western-Atlantic species that cross the MAB represent only 30 per cent of those that cross the AOP.
For the MAB, larval-development mode, maximum depth of occurrence and ability to live in brackish habitat did not add significant predicative power to the full GLMMs and were dropped from the final models (table 1a). For the AOP GLMMs, rafting ability, maximum depth of occurrence and ability to live in brackish habitat were dropped from the final models for the same reason (table 1a). Marginal predictor variables in the full models were retained in each final model: multi-habitat use for the MAB and maximum body size for the AOP (table 1a). There were no significant two-way interactions between predictor variables.
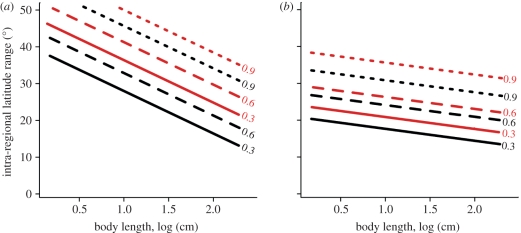
Significant positive predictors for overcoming both the MAB and the AOP were intra-regional latitudinal-range and maximum body size (table 1). Additional positive predictors for crossing one barrier were rafting ability for the MAB and multi-habitat use for the AOP. Larval-development mode was a significant predictor for the AOP in the full model, and was thus maintained in further analysis, but no significant differences were found in any pairwise tests between the three development modes in the final model (table 1b). For both the MAB and the AOP models, the intercepts were significantly different from zero, indicating that, while the significant traits increase the probability of barrier crossing, a lack of these traits does not mean that the probability is close to zero (figure 2).
Figure 2.
Line plots of predicted potential of overcoming each marine biogeographic barrier by Atlantic reef fishes as a function of their ecological traits. For visual simplicity, standard errors are omitted. Plots were derived from probability values produced by each GLMM (see table 1b). (a) Mid-Atlantic Barrier (MAB; black, rafter; red, non-rafter); (b) Amazon–Orinoco Plume (AOP; black, multi-habitat user; red, reef-habitats only). Rafter: juveniles/adults associate with flotsam; body size: maximum known length; multi-habitat user: associate with reef and other habitat types; intra-regional latitudinal-range: the largest range within either the Greater Caribbean or Brazilian Provinces.
The MAB and the AOP GLMMs that excluded data on species from super-disperser families produced the same patterns as the full models that included super-dispersers (electronic supplementary material, table S2). Large adult-size and rafting ability were significant predictors in all MAB models, including the GLMM for both East + West-Atlantic species that did not incorporate latitudinal-range as a factor.
4. Discussion
Range expansions of tropical reef fish species over two permeable marine barriers to dispersal within the tropical Atlantic are associated with somewhat different suites of ecological traits. A pelagic-dispersal mechanism (rafting) was linked to crossing only one barrier, the MAB. Interestingly, variation in larval-development mode, which could well have some effect on larval-dispersal potential, did not act as a significant predictor for crossing either the MAB or the AOP. These results indicate that, for a large oceanic barrier for which relatively long transit times can be expected, basic variation in larval-development mode has no general effect on trans-barrier occurrence, but the `increased dispersal potential provided by rafting does become important. Rafting may act not only as a pelagic-dispersal mechanism but also as an establishment enhancer. Rafting individuals enter a new area as juveniles or adults that have already passed through the early (larval) life-history stage of high mortality. Further, because flotsam is well known to act as an aggregator that attracts fishes, rafting individuals are more likely to arrive in groups. Both these factors could promote population establishment in a new area.
Pelagic larval duration (PLD) has been viewed as a convenient surrogate for dispersal potential in marine species that have a sedentary adult stage and pelagic larvae. However, empirical support for the generality of this relationship is lacking, notably in the tropical Atlantic [48]. Because estimates of species' PLD are available for relatively few species, larval-development modes have been used as broad indicators of larval-dispersal potential [21]. Our analyses indicate that variation in larval-development mode is not a significant predictor of crossing either the MAB or the AOP. Any effects of variation in larval-dispersal potential indicated by larval-development mode seem to have been overwhelmed by stronger effects of rafting, habitat use, body size and latitudinal-range.
Significant predictors for both the MAB and the AOP are large latitudinal-range and large adult-size. The probability of successful establishment following transit across a barrier, and the factors that affect that success, has not been formally considered as a factor affecting the large-scale distributions of tropical reef fishes in previous studies. Latitudinal-range may be associated with the degree of tolerance of varying environmental conditions by both demersal adults and pelagic larvae [49], and large native ranges are often considered to be good predictors of invasion success [50]. Eurytolerance of environmental conditions by adults and larvae, as indicated by large latitudinal-ranges, may facilitate establishment of species of tropical reef fishes that cross the MAB and the AOP.
A relationship between large adult size and both range-size and barrier crossing has been documented for both terrestrial and marine species [8,51,52]. The positive correlation we found between body size and occurrence on both sides of the MAB and the AOP may reflect an advantage that large-bodied species have in population establishment in novel habitat [53]. Body size has long been suggested as a trait associated with colonization success, mainly because it is linked to faster growth, greater competitive ability, enhanced predator avoidance and tolerance of environmental variability [9,29,53]. In addition, longevity broadly correlates positively with body size among fishes [54], and long-lived individuals may provide a ‘buffer’ against extinctions during the population-establishment phase resulting from long intervals between sporadic recruitment events across barriers [38,55]. In summary, adults of large-bodied tropical reef fish species may be better at colonizing new habitats and expanding their ranges across marine barriers.
Multi-habitat use was the only habitat factor related to crossing of the AOP but not the MAB by Western-Atlantic species, and neither maximum depth of occurrence nor use of brackish habitats had any effect on crossing the AOP. Habitat eurytolerance may affect crossing the AOP in two different ways: (i) it may allow adults to use non-reef habitats within the AOP as stepping stones to facilitate crossing, or (ii) the ability to use a range of habitats may enhance establishment after crossing. Multi-habitat use did not predict species' crossing the MAB. As it is hard to see how being able to use a variety of habitats would not facilitate establishment after crossing both barriers, we suggest that the inconsistency between the effects of multi-habitat use in the MAB and the AOP may arise because multi-habitat use facilitates AOP-crossing through a stepping-stone effect during crossing rather than an establishment effect following crossing.
Migration traffic is likely to occur in both directions, which is potentially indicated by rare occurrences of some species one side of a barrier or the other [17]. However, determining the direction that various species crossed a barrier, and how biological and ecological traits might vary with respect to movements in each direction, will require complicated and time-consuming genetic studies involving many taxa (cf. [56]). At present, all we can say is which factors are involved in assisting species to overcome each barrier, and that our analyses have detected factors unrelated to larval-dispersal potential that have not been previously been examined for reef fishes.
Crossing both the MAB and the AOP is associated with large latitudinal-ranges and large body size, while rafting is an additional factor for the MAB and multi-habitat use for the AOP. These differences relate to the relative ease with which each barrier can be crossed. The AOP alone provides non-reef habitat useable by adults of certain species of reef fishes to facilitate crossing, and is probably easier to cross by a variety of types of pelagic propagules because it is narrower and current speeds across it are faster (http://oceancurrents.rsmas.miami.edu/atlantic/atlantic.html). The effect of this combination of factors is that many more species of a broader range of taxa and ecotypes cross the AOP than the MAB. The great majority of rafting species that cross the MAB also cross the AOP, and in fact more species of rafters cross the AOP [51] than cross the MAB [37]. However, rafting is not an important factor at the assemblage level for crossing the AOP because rafters represent a much smaller proportion of AOP crossers (17.4%) than of MAB crossers (36.1%).
The two barriers we examined have idiosyncratic features that endow them with a certain measure of uniqueness and thus limit generalizations that can be made to other large marine barriers, such as those that affect tropical reef fishes in the Indo-Pacific. The AOP is formed by the world's largest river discharge system, whose freshwater output influences the longest such stretch of coastline in the world. In addition, the AOP deposits huge amounts of rainforest plant debris as flotsam into the Atlantic Ocean every year [2]. The effects of other, smaller discharge systems on Indo-Pacific reef fish faunas, which also differ in taxonomic composition to the Atlantic reef fish fauna, remain to be assessed. The MAB, on the other hand, probably is broadly equivalent to other wide oceanic barriers in the Indo-Pacific, such as the 4000+ km wide Eastern-Pacific Barrier (EPB) [6,56]. Rafting may have an important role in dispersal across the EPB [6]. However, the role of rafting may be greatly enhanced with respect to crossing the MAB owing to the coastal geography of the Atlantic Ocean. Large continental landmasses on both sides of that barrier provide abundant potential sources of plant debris that form the floating substrata for rafting fishes, plus large targets where drifting material can deliver rafting reef fishes. By contrast, the EPB has a large land mass only on one side, and a few small, highly scattered islands on the other. Factors that could facilitate pelagic dispersal over the EPB were analysed separately by Robertson et al. [6], and no attempt was made to account for correlations between them. A reassessment of dispersal by transpacific shore fishes across the EPB that models a combination of a similar suite of factors to those we considered for the MAB and the AOP should provide a test of the generality of our findings. Finally, although our analyses were restricted to tropical fish assemblages, circulating ocean currents connect the eastern and western sides of both the North and the South Atlantic temperate zones. Further analysis on ecological traits of temperate fish and their distribution on either side of the Atlantic using a similar approach should present an opportunity to test hypotheses accounting for latitudinal differences in dispersal ability [8].
5. Conclusions
Our analyses of tropical Atlantic reef fishes clearly indicate that assessments of factors which influence the successful crossing of marine barriers that focus on variation in larval life histories as determinants of pelagic-dispersal ability, will be far from adequate for the many shore organisms that produce pelagic larvae. Inclusion of a broader range of pelagic-dispersal and adult-biology factors in our analyses relegated variation in major modes of larval-development to an insignificant role as a determinant of large barrier crossing by these fishes. Flotsam-rafting provides extra capacity to the pelagic-dispersal mechanism as well as possibly enhancing post-crossing establishment, and plays a substantial role in determining distributions relative to a large deep-water oceanic barrier. Multiple-habitat use may facilitate expansion over long stretches of coastal adverse conditions through a stepping-stone effect. Finally, adult and larval eurytolerance, as indicated by large size and large latitudinal-range, may influence faunal patterns of crossing large barriers by enhancing establishment. Future assessments of factors influencing the distributions of demersal marine organisms relative to large marine barriers must broaden their focus to include various pelagic-dispersal mechanisms, and factors that influence whether migrants successfully establish populations in the new habitat.
Acknowledgements
Financial support was provided by an International Macquarie University Research Excellence Scholarship (O.J.L.), the Australian Research Council (J.S.M), the Smithsonian Tropical Research Institute (D.R.R.; STV grant to S.R.F.), the National Geographic Society (grant 7937-05 to S.R.F) and the CNPq (S.R.F.). This work benefited from informal meetings at the National Centre for Ecological Analysis and Synthesis, a centre funded by the NSF (grant DEB-0072909) and the University of California, Santa Barbara. We thank Michel Kulbicki and five anonymous reviewers for suggestions on the manuscript.
References
- 1.Palumbi S. R. 1994. Genetic divergence, reproductive isolation, and marine speciation. Annu. Rev. Ecol. Syst. 25, 547–572 10.1146/annurev.es.25.110194.002555 (doi:10.1146/annurev.es.25.110194.002555) [DOI] [Google Scholar]
- 2.Rocha L. A. 2003. Patterns of distribution and processes of speciation in Brazilian reef fishes. J. Biogeogr. 30, 1161–1171 10.1046/j.1365-2699.2003.00900.x (doi:10.1046/j.1365-2699.2003.00900.x) [DOI] [Google Scholar]
- 3.Floeter S. R., et al. 2008. Atlantic reef fish biogeography and evolution. J. Biogeogr. 35, 22–47 10.1111/j.1365-2699.2007.01790.x (doi:10.1111/j.1365-2699.2007.01790.x) [DOI] [Google Scholar]
- 4.Briggs J. C. 1974. Operation of zoogeographic barriers. Syst. Zool. 23, 248–256 10.2307/2412136 (doi:10.2307/2412136) [DOI] [Google Scholar]
- 5.Joyeux J. C., Floeter S. R., Ferreira C. E. L., Gasparini J. L. 2001. Biogeography of tropical reef fishes: the South Atlantic puzzle. J. Biogeogr. 28, 831–841 10.1111/j.1365-2699.2001.00602.x (doi:10.1111/j.1365-2699.2001.00602.x) [DOI] [Google Scholar]
- 6.Robertson D. R., Grove J. S., McCosker J. E. 2004. Tropical transpacific shore fishes. Pac. Sci. 58, 507–565 10.1353/psc.2004.0041 (doi:10.1353/psc.2004.0041) [DOI] [Google Scholar]
- 7.Thorrold S. R., McKinnon A. D. 1995. Response of larval fish assemblage to a riverine plume in coastal waters of the Great Barrier Reef lagoon. Limnol. Oceanogr. 40, 177–181 10.4319/lo.1995.40.1.0177 (doi:10.4319/lo.1995.40.1.0177) [DOI] [Google Scholar]
- 8.Bradbury I. R., Laurel B., Snelgrove P. V. R., Bentzen P., Campana S. E. 2008. Global patterns in marine dispersal estimates: the influence of geography, taxonomic category and life history. Proc. R. Soc. B 275, 1803–1809 10.1098/rspb.2008.0216 (doi:10.1098/rspb.2008.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeij G. J. 2004. Island life: a view from the sea. In Frontiers of biogeography: new directions in the geography of nature (eds Lomolino M. V., Heaney L. R.), pp. 239–254 Sunderland, MA: Sinauer Associates [Google Scholar]
- 10.Briggs J. C. 1995. Global biogeography. New York, NY: Elsevier [Google Scholar]
- 11.Bellwood D. R., Wainwright P. C. 2002. The history and biogeography of fishes on coral reefs. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale P.), pp. 3–15 San Diego, CA: Academic Press [Google Scholar]
- 12.Rocha L. A., Bass A. L., Robertson D. R., Bowen B. W. 2002. Adult habitat preferences, larval dispersal, and the comparative phylogeography of three Atlantic surgeonfishes (Teleostei: Acanthuridae). Mol. Ecol. 11, 243–252 10.1046/j.0962-1083.2001.01431.x (doi:10.1046/j.0962-1083.2001.01431.x) [DOI] [PubMed] [Google Scholar]
- 13.Rocha L. A., Robertson D. R., Rocha C. R., Van Tassell J. L., Craig M. T., Bowen B. W. 2005. Recent invasion of the tropical Atlantic by an Indo-Pacific coral reef fish. Mol. Ecol. 14, 3921–3928 10.1111/j.1365-294X.2005.02698.x (doi:10.1111/j.1365-294X.2005.02698.x) [DOI] [PubMed] [Google Scholar]
- 14.Rocha L. A., Rocha C. R., Robertson D. R., Bowen B. W. 2008. Comparative phylogeography of Atlantic reef fishes indicates both origin and accumulation of diversity in the Caribbean. BMC Evol. Biol. 8, 157. 10.1186/1471-2148-8-157 (doi:10.1186/1471-2148-8-157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowen B. W., Bass A. L., Muss A. J., Carlin J., Robertson D. R. 2006. Phylogeography of two Atlantic squirrelfishes (family Holocentridae): exploring pelagic larval duration and population connectivity. Mar. Biol. 149, 899–913 10.1007/s00227-006-0252-1 (doi:10.1007/s00227-006-0252-1) [DOI] [Google Scholar]
- 16.Robertson D. R., Karg F., Moura R. L., Victor B. C., Bernardi G. 2006. Mechanisms of speciation and faunal enrichment in Atlantic parrotfishes. Mol. Phyl. Evol. 40, 795–807 10.1016/j.ympev.2006.04.011 (doi:10.1016/j.ympev.2006.04.011) [DOI] [PubMed] [Google Scholar]
- 17.Luiz O. J., Jr, Floeter S. R., Gasparini J. L., Ferreira C. E. L., Wirtz P. 2004. The occurrence of Acanthurus monroviae (Perciformes: Acanthuridae) in the southwestern Atlantic, with comments on other eastern Atlantic reef fishes occurring in Brazil. J. Fish Biol. 65, 1173–1179 10.1111/j.1095-8649.2004.00519.x (doi:10.1111/j.1095-8649.2004.00519.x) [DOI] [Google Scholar]
- 18.Collette B. B., Rützler K. 1977. Reef fishes over sponge bottoms off the mouth of the Amazon River. In Proc. 3rd Int. Coral Reef Symp., vol. 1, pp. 305–310 Miami, FL: International Society for Reef Studies. [Google Scholar]
- 19.Thresher R. E. 1991. Geographic variability in the ecology of coral reef fishes: evidence, evolution and possible implications. In The ecology of fishes on coral reefs (ed. Sale P. F.), pp. 401–436 San Diego, CA: Academic Press [Google Scholar]
- 20.Floeter S. R., Gasparini J. L. 2000. The southwestern Atlantic reef-fish fauna: composition and zoogeographic patterns. J. Fish Biol. 56, 1099–1114 10.1111/j.1095-8649.2000.tb02126.x (doi:10.1111/j.1095-8649.2000.tb02126.x) [DOI] [Google Scholar]
- 21.Hughes T. P., Bellwood D. R., Connolly S. R. 2002. Biodiversity hotspots, centres of endemicity, and the conservation of coral reefs. Ecol. Lett. 5, 775–784 10.1046/j.1461-0248.2002.00383.x (doi:10.1046/j.1461-0248.2002.00383.x) [DOI] [Google Scholar]
- 22.Leis J. M., McCormick M. I. 2002. The biology, behavior, and ecology of the pelagic, larval stage of coral reef fishes. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale P.), pp. 3–15 San Diego, CA: Academic Press [Google Scholar]
- 23.Jokiel P. L. 1990. Long-distance dispersal by rafting: reemergence of an old hypothesis. Endeavour 14, 66–73 10.1016/0160-9327(90)90074-2 (doi:10.1016/0160-9327(90)90074-2) [DOI] [Google Scholar]
- 24.Thiel M., Gutow L. 2005. The ecology of rafting in the marine environment. II. The rafting organisms and community. Oceanogr. Mar. Biol. 43, 279–418 10.1201/9781420037449.ch7 (doi:10.1201/9781420037449.ch7) [DOI] [Google Scholar]
- 25.Fraser C. I., Nikula R., Waters J. M. 2010. Oceanic rafting by a coastal community. Proc. R. Soc. B 277, 649–655 10.1098/rspb.2010.1117 (doi:10.1098/rspb.2010.1117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teske P. R., et al. 2005. Molecular evidence for long-distance colonization in an Indo-Pacific seahorse lineage. Mar. Ecol. Prog. Ser. 286, 249–260 10.3354/meps286249 (doi:10.3354/meps286249) [DOI] [Google Scholar]
- 27.Brown J. H. 1995. Macroecology. Chicago, IL: University of Chicago Press [Google Scholar]
- 28.Reaka M. L., Rodgers P. J., Kudla A. U. 2008. Patterns of biodiversity and endemism on Indo-West Pacific coral reefs. Proc. Natl Aacd. Sci. USA 105, 11 474–11 481 10.1073/pnas.0802594105 (doi:10.1073/pnas.0802594105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermeij G. J., Dietl G. P., Reid D. G. 2008. The trans-Atlantic history of diversity and body size in ecological guilds. Ecology 89(Suppl. 11), S39–S52 10.1890/07-0663.1 (doi:10.1890/07-0663.1) [DOI] [PubMed] [Google Scholar]
- 30.Carpenter K. E. 2002. The living marine resources of the Western Central Atlantic, vol 3. FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication. Rome, Italy: Food and Agriculture Organisation of the United Nations.
- 31.Böhlke J. E., Chaplin C. C. G. 1993. Fishes of the Bahamas and adjacent tropical waters. Austin, TX: University of Texas Press [Google Scholar]
- 32.Randall J. E. 1996. Caribbean reef fishes, 3rd edn. Neptune City, NJ: TFH Publications [Google Scholar]
- 33.Smith C. 1997. Tropical marine fishes of the Caribbean, the Gulf of Mexico, Florida, the Bahamas and Bermuda. New York, NY: Alfred A. Knopf, Inc [Google Scholar]
- 34.Carvalho-Filho A. 1999. Peixes: Costa Brasileira, 3rd edn. São Paulo, Brazil: Editora Melro [Google Scholar]
- 35.Feitoza B. M., Rosa R. S., Rocha L. 2005. Ecology and zoogeography of deep reef fishes in northeastern Brazil. Bull. Mar. Sci. 76, 725–742 [Google Scholar]
- 36.Castro J. J., Santiago J. A., Santana-Ortega A. T. 2002. A general theory on fish aggregation to floating objects: an alternative to the meeting point hypothesis. Rev. Fish Biol. Fish. 11, 255–277 10.1023/A:1020302414472 (doi:10.1023/A:1020302414472) [DOI] [Google Scholar]
- 37.Bradshaw C. J. A., Brook B. W. 2010. The conservation biologist's toolbox: principles for the design and analysis of conservation studies. In Conservation biology for all (eds Sodhi N. S., Ehrlich P. R.), pp. 313–340 Oxford, UK: Oxford University Press [Google Scholar]
- 38.Mellin C., Huchery C., Caley M. J., Meekan M. G., Bradshaw C. J. A. 2010. Reef size and isolation determine the temporal stability of coral reef fish populations. Ecology 91, 3138–3145 10.1890/10-0267.1 (doi:10.1890/10-0267.1) [DOI] [PubMed] [Google Scholar]
- 39.Lee T. M., Jetz W. 2011. Unravelling the structure of species extinction risk for predictive conservation science. Proc. R. Soc. B 278, 1329–1338 10.1098/rspb.2010.1877 (doi:10.1098/rspb.2010.1877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reece J. S., Bowen B. W., Joshi K., Goz V., Larson A. 2010. Phylogeography of two moray eels indicates high dispersal throughout the Indo-Pacific. J. Hered. 101, 391–402 10.1093/jhered/esq036 (doi:10.1093/jhered/esq036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venables W. N., Ripley B. D. 2002. Modern applied statistics with S, 4th edn. New York, NY: Springer [Google Scholar]
- 42.Zuur A. F., Ieno E. N., Walker N. J., Saveliev A. A., Smith G. M. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer [Google Scholar]
- 43.Thomson F. J., Moles A. T., Auld T. D., Ramp D., Ren S., Kingsford R. T. 2010. Chasing the unknown: predicting seed dispersal mechanisms from plant traits. J. Ecol. 98, 1310–1318 10.1111/j.1365-2745.2010.01724.x (doi:10.1111/j.1365-2745.2010.01724.x) [DOI] [Google Scholar]
- 44.Pinheiro J., Bates D. 2000. Mixed-effects models in S and S-PLUS. New York, NY: Springer [Google Scholar]
- 45.Krackow S., Tkadlec E. 2001. Analysis of brood sex ratios: implications of offspring clustering. Behav. Ecol. Sociobiol. 50, 293–301 10.1007/s002650100366 (doi:10.1007/s002650100366) [DOI] [Google Scholar]
- 46.Bronstöm G. 2009. glmmML: generalized linear models with clustering. R package version 0.81-6. See http://CRAN.R-project.org/package=glmmML
- 47.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.Rproject.org [Google Scholar]
- 48.Lester S. E., Ruttenberg B. I. 2005. The relationship between pelagic larval duration and range size in tropical reef fishes: a synthetic analysis. Proc. R. Soc. B 272, 585–591 10.1098/rspb.2004.2985 (doi:10.1098/rspb.2004.2985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones G. P., Caley M. J., Munday P. L. 2002. Rarity in coral reef fish communities. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale P.), pp. 81–101 San Diego, CA: Academic Press [Google Scholar]
- 50.Lodge D. M. 1993. Biological invasions: lessons for ecology. Trends Ecol. Evol. 8, 133–137 10.1016/0169-5347(93)90025-K (doi:10.1016/0169-5347(93)90025-K) [DOI] [PubMed] [Google Scholar]
- 51.Reaka M. L. 1980. Geographic range, life history patterns, and body size in a guild of coral-dwelling mantis shrimps. Evolution 34, 1019–1030 10.2307/2408010 (doi:10.2307/2408010) [DOI] [PubMed] [Google Scholar]
- 52.Jenkins D. G., et al. 2007. Does size matter for dispersal distance? Glob. Ecol. Biogeogr. 16, 415–425 10.1111/j.1466-8238.2007.00312.x (doi:10.1111/j.1466-8238.2007.00312.x) [DOI] [Google Scholar]
- 53.Roy K., Jablonski D., Valentine J. W. 2002. Body size and invasion success in marine bivalves. Ecol. Lett. 5, 163–167 10.1046/j.1461-0248.2002.00316.x (doi:10.1046/j.1461-0248.2002.00316.x) [DOI] [Google Scholar]
- 54.Helfman G. S., Collette B. B., Facey D. E., Bowen B. W. 2009. The diversity of fishes. Oxford, UK: Wiley-Blackwell [Google Scholar]
- 55.Robertson R., Kaufmann K. W. 1998. Assessing early recruitment dynamics and its demographic consequences among tropical reef fishes: accommodating variation in recruitment seasonality and longevity. Aust. J. Ecol. 23, 226–233 10.1111/j.1442-9993.1998.tb00724.x (doi:10.1111/j.1442-9993.1998.tb00724.x) [DOI] [Google Scholar]
- 56.Lessios H. A., Robertson D. R. 2006. Crossing the impassable: genetic connections in 20 reef fishes across the Eastern Pacific Barrier. Proc. R. Soc. B 273, 2201–2208 10.1098/rspb.2006.3543 (doi:10.1098/rspb.2006.3543) [DOI] [PMC free article] [PubMed] [Google Scholar]