Abstract
How and why diverse sexual systems evolve are fascinating evolutionary questions, but few empirical studies have dealt with these questions in animals. Pedunculate (gooseneck) barnacles show such diversity, including simultaneous hermaphroditism, coexistence of dwarf males and hermaphrodites (androdioecy), and coexistence of dwarf males and females (dioecy). Here, we report the first phylogenetically controlled test of the hypothesis that the ultimate cause of the diverse sexual systems and presence of dwarf males in this group is limited mating opportunities for non-dwarf individuals, owing to mating in small groups. Within the pedunculate barnacle phylogeny, dwarf males and females have evolved repeatedly. Females are more likely to evolve in androdioecious than hermaphroditic populations, suggesting that evolution of dwarf males has preceded that of females in pedunculates. Both dwarf males and females are associated with a higher proportion of solitary individuals in the population, corroborating the hypothesis that limited mating opportunities have favoured evolution of these diverse sexual systems, which have puzzled biologists since Darwin.
Keywords: androdioecy, dwarf male, mating system, phylogeny, sexuality pattern
1. Introduction
Diverse sexual systems in organisms and the evolutionary forces driving them have been a major topic of inquiry in evolutionary biology [1–7]. However, few empirical studies have addressed these problems, especially in animals. Interest in sexual systems dates back to Darwin [8], who found that pedunculate barnacles (Thoracica: Pedunculata) show such diversity, including simultaneous hermaphroditism, coexistence of males and hermaphrodites (often called androdioecy), and coexistence of males and females (dioecy; figure 1). Darwin also found that barnacle males are always much smaller than mature hermaphrodites or females [9–11] and that these males (referred to as dwarf males) are attached to specific sites of large conspecifics [12]. However, he did not specify the evolutionary force responsible for this sexual diversity and the occurrence of dwarf males. He wrote that ‘the diversity in sexual relations … appears to me eminently curious’ (p. 292 of [8]), but that ‘regarding the final cause … of separation of the sexes … and … of the existence of Complemental males (i.e. dwarf males attached to hermaphrodites), I can throw no light’ (p. 291).
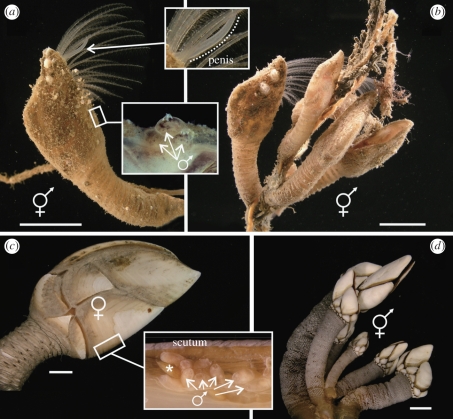
Figure 1.
Three scalpellid species representing different sexual systems. (a,b) Scalpellum scalpellum. Androdioecious species attached to hydroids. (a) A solitary hermaphrodite (note the penis; upper inset) that depends on dwarf males (lower inset) for reproduction. (b) A small group that can reproduce by cross-fertilization even without dwarf males. (c) Trianguloscalpellum regium. This deep-sea dioecious species depends entirely on the presence of dwarf males for fertilization. The receptacle inside the scutal edge (inset) contains numerous males (asterisk indicates a metamorphosing cyprid). (d) Pollicipes pollicipes. This hermaphroditic species inhabits the upper rocky intertidal zone in dense populations, which ensures cross-fertilization. Scale bar, 1 cm.
Although the ultimate cause of the diverse sexual systems in barnacles has been an enigma for more than a century, recent theoretical studies have suggested that both the evolution of separate sexes and male dwarfing can be understood under sex allocation models [2,13–15]. Since barnacles are sedentary animals that mate with conspecifics within the reach of their penises, male fitness is limited by the number of available eggs produced by neighbouring individuals, following the law of diminishing returns [2,13–15]. Thus, total fitness through both male and female functions is maximized at an intermediate allocation to both functions, making simultaneous hermaphroditism evolutionarily stable. However, as population density decreases, these theories predict that the optimal allocation to male function (sperm production) of each hermaphrodite becomes smaller, and the intensity of sperm competition is reduced. Then dwarf males, even with a small amount of sperm, can expect some fertilization success although they must compete with hermaphrodites for fertilizing eggs. Since dwarf males have an advantage of surviving better to maturity [13,15] and possibly a mating advantage by attaching nearer the fertilization site of conspecifcs [1,16], they may have fitness comparable to that of hermaphrodites, and hence are expected to evolve. The evolutionarily stable proportion of larvae that become dwarf males increases as the population density reduces, but it does not exceed 50 per cent [15]. When density becomes so low that most individuals live solitarily and have no chance to fertilize conspecifics, they should allocate all resources to female function to produce as many eggs as possible and become pure females. These females are expected to be fertilized by dwarf males as most females keep more than one male [12,17]. In fact, in the best-studied pedunculate, Scalpellum scalpellum, although androdioecious, both genetic and environmental factors affect sex determination, and the proportion of larvae that will develop into males does not exceed 50 per cent [18]. However, two to five dwarf males are usually attached to a large hermaphrodite in the field [12], because of much earlier sexual maturity of males (within 10 days after settlement) than hermaphrodites (at least 1 year) [18]. Thus, the sex allocation models on barnacle sexuality [2,13–15] predict that (i) as mating opportunities become more limited, both dwarf males and females are expected to evolve, and (ii) dwarf males will evolve prior to females as the density decreases (i.e. they tend to evolve in hermaphroditic populations, whereas females tend to evolve in androdioecious populations). Gynodioecy (populations with females and hermaphrodites) is predicted to be extremely rare. Although mode of sex determination is largely unknown in barnacles, the above models postulate genetically determined strategies [13–15]. However, sex allocation strategies are usually unrelated to the underlying mechanisms [2] (see §4).
Most shallow-water pedunculate barnacles that live in large groups, such as Pollicipes or Lepas spp., are hermaphroditic [19], whereas dwarf males and pure females tend to occur in deep sea or symbiotic forms [20,21] (figure 1). To critically test the importance of limited mating opportunities on the reproductive mode of barnacles, we collected information on sexual systems and the extent of limitation of mating opportunities in 48 pedunculate barnacle species. The degree of limitation was assessed as the proportion of solitary non-dwarf individuals in a sampled barnacle population. Since species are not independent units of comparison, phylogenetic information is required for testing character evolution and factors affecting it [22,23]. Therefore, molecular phylogenies of these species were constructed using approximately 1400 bp of 18S rDNA from published sources [24,25] and our own sequencing results (electronic supplementary material, table S1).
2. Material and methods
(a). Sexual systems
The sexual system of each species was studied by dissection of samples or coded based on literature data (electronic supplementary material, table S1). Although ‘true’ barnacles (superorder Thoracica) comprise two orders, Pedunculata (pedunculate barnacles) and Sessilia (acorn barnacles), we confined our analyses to the pedunculates because few dwarf males and no females have been observed in acorn barnacles [19,26–30], which may imply phylogenetic constraint. In addition, unlike pedunculates, the penises of some acorn barnacles are lost in non-reproductive seasons [31], rendering the distinction between hermaphrodites and females inaccurate. Large (non-dwarf) individuals were recorded as hermaphrodites if they had a penis and as females if they lacked a penis. Dwarf males were defined as males that mature (as judged by the presence of a developed penis or testis) at a much smaller size (less than half as long) than conspecific hermaphrodites or females [10,11]. No large males are known in barnacles, but dwarf males of several barnacle species continue to grow, and some of them may later become hermaphroditic [11,26,30]. They differ from normal hermaphrodites in that (i) they are always attached to specific sites of conspecifics, (ii) they allocate more resources to male function and (iii) only a few (if any) will become hermaphroditic [11,19]. In this paper, the coexistence of such dwarf males and hermaphrodites is also referred to as androdioecy, although androdioecy normally refers to the coexistence of pure males and hermaphrodites [32,33]. A species was regarded as hermaphroditic (as opposed to androdioecious) if no males were found or reported even after several non-dwarf individuals were investigated, irrespective of the sexual system of the majority of the lineage. For instance, Annandale [34] reported that Arcoscalpellum sociable are ‘so gregarious’ but ‘no males were found’, and that in Trianguloscalpellum balanoides ‘males are believed to be always absent’. Thus, these species are treated as hermaphroditic.
(b). Barnacle distribution pattern
Data on the proportion of solitary individuals were obtained either from our own observations or from the literature (electronic supplementary material, table S1). Although mating group size is usually referred to as a factor that determines male fitness curves [2,13], group size is often difficult to measure in practice (the number of individuals was often simply recorded as ‘several’ or ‘many’ in the literature). A solitary individual was defined as a non-dwarf individual that had no neighbouring conspecifics (except for dwarf males) within the distance that it could fertilize. This distance is approximately twice its total length [35]. When accurate data on the distance between individuals were not available, individuals were regarded as being solitary only when they were attached singly to a specific substratum (such as molluscan shells, pebbles, corals, crabs, etc.).
(c). DNA amplification and sequencing
Total genomic DNA was extracted from muscle tissue of barnacles using the QuickGene DNA tissue kit (Fujifilm). PCR products for the 18S rDNA gene were amplified using the primers 18S 1.2F and 18S b5.0, 18S a0.7 and 18S b2.5, 18S a1.0 and 18S bi, and 18S a3.5 and 18S 9R, as described by Whiting [36], under the following temperature regime: an initial denaturation at 96°C for 3 min, followed by 35 cycles of 95°C for 1 min, 45°C–50°C for 30 s and 72°C for 1 min, followed by an extension at 72°C for 7 min. Amplification products were purified using ExoSAP-IT (USB Corporation), and then sequenced using the ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit on an ABI Prism 310 Genetic Analyzer or an ABI Prism 3130xl Genetic Analyzer. All final sequences were obtained from both strands for verification.
(d). Phylogenetic analysis
Phylogenetic analyses were performed using the sequence data (approx. 1400 bp) from 18S ribosomal DNA (18S rDNA) obtained to date from public genetic databases (GenBank, DDBJ, etc.) and from our own sequencing results (see electronic supplementary material, table S1 for accession numbers). Sequence data for a total of 48 species were used for the phylogenetic analysis. The 48 species represent all four suborders and 10 of 14 families of the order Pedunculata, excluding the small families of Anelasmatidae (consisting of one sp.), Malacolepadidae (two spp.), Microlepadidae (three spp.) and Rhizolepadidae (two spp.). Sequences were initially aligned using ClustalX [37] with default gap penalties, and regions of uncertain homology were removed before phylogenetic analysis. Phylogenetic relationships were analysed by Bayesian method as implemented by MrBayes v. 3.1.2 [38], and by maximum-likelihood (ML) analyses using the software package PAUP* v. 4.0b10 [39]. Sequences were first analysed with Modeltest software [40] to find the evolutionary model that best fitted the data. The Bayesian analyses were performed by running a Markov chain Monte Carlo (MCMC) algorithm for 10 000 000 cycles, sampling every 2000th generation. The initial 40 per cent of cycles were discarded for tree-building, and the convergence of MCMC runs was confirmed by MrBayes. The posterior probabilities of the phylogeny were determined by constructing a 50 per cent majority-rule consensus of the remaining 3000 trees. The ML analysis was performed using heuristic searches, with 10 random additional replicates and TBR branch-swapping. One hundred replications, TBR branch-swapping and one random addition replicate were used for bootstrap analysis.
(e). Statistical tests using Bayesian inference
To conduct Bayesian tests, 3000 Bayesian trees for constructing the consensus tree were used. Since BayesTraits cannot treat polytomies (three or more unresolved branches from a common ancestor), the trees were re-rooted with both Ibla quadrivalvis and Ibla cumingi (Iblomorpha) always designated as the outgroup. The presence (1) and absence (0) of dwarf males and females were both treated as continuous variables [41] since the explanatory variable (the proportion of solitary individuals) was also continuous (BayesTraits does not allow mixture of continuous and discrete variables). Their relationships were analysed using the Continuous module in BayesTraits (http://www.evolution.rdg.ac.uk/BayesTraits.html) [22,23].
An MCMC algorithm was run for 5 050 000 cycles, sampling every 100th generation, and the initial 50 000 cycles were discarded (all default settings of BayesTraits). The log-likelihoods of the MCMC samples from the unrestricted model were compared with those of the model that restricts the covariance to 0 [42]. The log Bayes factor, which is two times the difference between the natural logarithms of the harmonic means of the likelihoods of the two models, was used as the test statistic [42] (see also the manual of BayesTraits, available at the website). The logic is similar to likelihood-ratio test, but it uses the marginal likelihoods of the models rather than MLs. In the log-scale, Bayes factors greater than 2 are considered positive evidence, and values greater than 5 are taken as strong evidence [42,43]. The scaling parameters kappa, delta and lambda for continuous variables (related to the tempo, mode and phylogenetic associations of trait evolution; see the discussion in the manual of Continuous) were simultaneously estimated. Other parameters were all set to the default values.
Transition rates among sexual systems were analysed using the Discrete module in BayesTraits. For this purpose, the presence and absence of dwarf males and females were treated as discrete variables. MCMC was run with RateDev (deviation of the normal distribution, from which changes to the rates are drawn) set at 30 to meet the criterion of the acceptance rate being approximately 0.2. Other parameters were all set to the default values. The harmonic mean of likelihoods of the unrestricted model was compared with that of the model that restricts q12 = q34 or q13 = q24. The likelihood ratio of the log harmonic means was compared to calculate the log Bayes factor. All analyses using Bayesian inference were run at least three times and confirmed that the statistical results did not differ among the runs.
(f). Statistical tests using maximum likelihood
ML [22,23] was also used to test the relationship between sexual systems and the proportion of solitary individuals based on the ML tree (electronic supplementary material, figure S1). Since the ML tree contained several polytomies, 100 trees with randomly resolved polytomies were created using Mesquite v. 2.71 [44] with branch lengths of 0.0001 [41,45]. The 100 trees gave very similar log-likelihoods, in the case of both the unrestricted (i.e. the variables were allowed to covary) and the restricted (covariance set to 0) models (with standard errors smaller than 0.001). The ranges of likelihood ratios of these models based on the 100 trees are shown in §3, as well as their p-values under the χ2 distribution with d.f. = 1 and α = 0.05.
Transition rates among sexual systems were analysed by ML using the Discrete module in BayesTraits, with the presence or absence of dwarf males and females treated as discrete variables. The log-likelihoods of the unrestricted model and those of the model that restricts q12 = q34 or q13 = q24 were calculated based on the 100 randomly resolved trees. The likelihood ratios of the 100 trees were each tested against the χ2 distribution with d.f. = 1 and α = 0.05, and their p-values are shown as ranges in §3.
3. Results
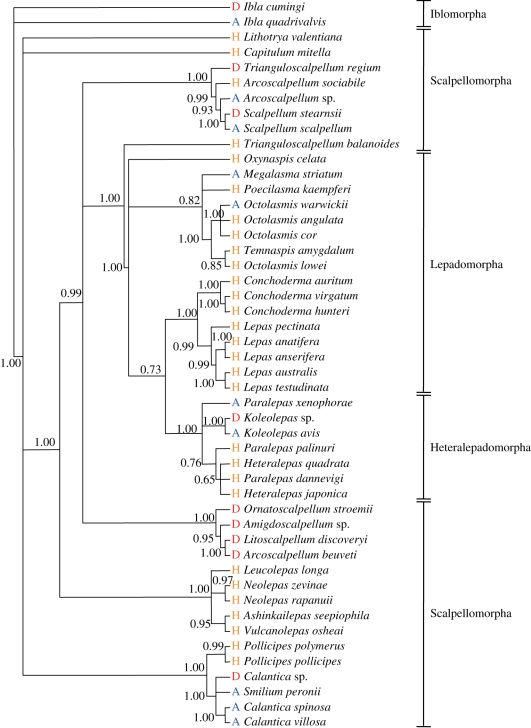
The obtained Bayesian (figure 2) and ML trees (electronic supplementary material, figure S1) are generally congruent with each other and with published trees [24,25,46]. The pattern of distribution of sexual systems on the Bayesian tree (figure 2) shows that dwarf males and females have multiple origins in the barnacle phylogeny (i.e. they have evolved independently in several lineages) [19]. Sexual systems differ even between closely related species, such as between Octolasmis angulata (hermaphroditic) and Octolasmis warwickii (androdioecious) [11] or between Koleolepas avis (androdioecious) and Koleolepas sp. (dioecious). Thus, sexuality can change on relatively short evolutionary timescales. The same conclusions were drawn from the ML tree (electronic supplementary material, figure S1).
Figure 2.
Sexual system placed on the Bayesian phylogenetic tree of pedunculate barnacles. The consensus tree was constructed with Bayesian inference based on the general time reversible model (GTR + I + G). Ibla cumingi and I. quadrivalvis were used as outgroups. Only confidence values higher than 0.5 are shown in the tree. H, hermaphroditic; A, androdioecious (males + hermaphrodites); D, dioecious (males + females).
When Bayesian inference [22,23] was used to test character evolution based on the Bayesian phylogeny (figure 2), there was a strong positive association between the proportion of solitary individuals and the presence of dwarf males (log Bayes factor statistics of correlated evolution = 23.14). Thus, as the proportion of solitary individuals increases, the probability of the presence of dwarf males increases. Likewise, there was also a positive association between the proportion of solitary individuals and the presence of females (log Bayes factor = 17.85). Virtually identical results were obtained if character evolution was tested based on the ML tree (electronic supplementary material, figure S1; likelihood ratio χ2 of 100 randomly resolved trees = 16.61–17.84, all p < 0.0001 for dwarf males; likelihood ratio χ2 = 18.64–19.55, all p < 0.0001 for females).
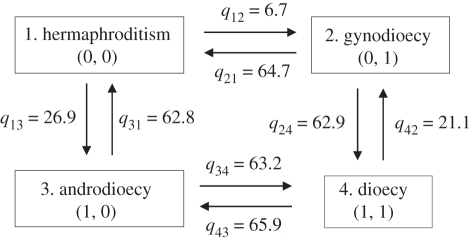
In both Bayesian (figure 2) and ML (electronic supplementary material, figure S1) trees, androdioecy occurs commonly, whereas no gynodioecy appears. Thus, the Bayesian inference on character coevolution (figure 3) suggests that evolutionary transition rates from the absence to the presence of females when dwarf males are lacking (i.e. from hermaphroditism to gynodioecy; q12 = 6.7) were much lower than the rates for moving from the absence to the presence of females when dwarf males are available (from androdioecy to dioecy; q34 = 63.2; log Bayes factor = 5.17). On the other hand, transition rates from the absence to the presence of dwarf males in the absence of females (from hermaphroditism to androdioecy; q13 = 26.9) did not differ significantly from the corresponding rates in the presence of females (from gynodioecy to dioecy; q24 = 62.9; log Bayes factor = 0.61). Although the evolutionary transition rates estimated by the ML method were different from those estimated using Bayesian inference (electronic supplementary material, figure S2), the above statistical conclusions were also supported (q12 versus q34: likelihood ratio χ2 of 100 randomly resolved trees = 4.35–4.56; p = 0.033–0.037; q13 versus q24: likelihood ratio χ2 = 0.65–0.83; p = 0.36–0.42). Taken together, these results suggest that the evolution of dwarf males preceded that of females in the transition of sexual systems from hermaphroditism to dioecy.
Figure 3.
Evolutionary transition rates between sexual systems in pedunculate barnacles estimated by Bayesian inference. qij refers to the transition rate from the ith to the jth sexual system. In parentheses, the presence (1) or absence (0) of dwarf males (left) and of females (right) is shown.
4. Discussion
Our results support the hypothesis that limitation of mating opportunities is important for the evolution of dwarf males (i.e. androdioecy) and females (i.e. dioecy) from hermaphrodites in pedunculate barnacles. This perspective contrasts with the widely accepted view that low density promotes hermaphroditism rather than dioecy to facilitate self-fertilization and/or mating with any mates individuals may encounter (low-density model) [1]. In pedunculate barnacles, these merits of being hermaphroditic are small, as they do not usually self-fertilize [47] and encounters with novel individuals are limited owing to their sedentary nature. Instead, as explained, sex allocation theories predict the evolution of hermaphroditism from dioecy (with normal-sized males), then androdioecy with dwarf males, and finally dioecy with dwarf males, as density decreases [2,13–15]. In addition, barnacle larvae (the cyprids) are highly mobile and actively select a suitable site for settlement [48]. This active settlement might have facilitated accurate and prompt settlement by larvae on the specific sites of large conspecifics, hence the evolution of dwarf males. Ghiselin [1] himself suggested that the combination of low density and the low mobility (or even sedentary) nature of large adults, together with highly mobile larvae or young adults, tends to promote the evolution of dwarf males. The likely examples include the sister taxa of ‘true’ (thoracican) barnacles (the parasitic Rhizocephala and the burrowing Acrothoracica), Cycliophora (sessile animals on lobster mouthparts), many parasitic Copepoda, Annelida, parasitic Gastropoda (Enteroxididae) and deep-sea angler fishes [1,10].
Sex allocation models usually postulate genetically determined strategies [2,4,5], and models on barnacle sexual systems are no exceptions [13–15]. Although there is evidence of genetic sex determination in some thoracican barnacles [18,27], the genetic basis of sexual systems is largely unknown and this may differ between barnacle species. However, the evolutionary consequences of sex allocation are largely unrelated to the underlying mechanisms [2,32]. Therefore, we believe that the general trend towards dioecy under low densities should apply also in the case of environmentally determined sexual systems, as conditions for the appearance of dwarf males and females (i.e. small mating groups and being solitary, respectively) are irrespective of whether underlying mechanisms of sexual systems are genetic or environmental. For instance, under plastic sexual expression, a larva that finds a solitary large individual should settle on it and become a dwarf male to fertilize its eggs as soon as possible [1]. However, a larva that settles in the middle of many large hermaphrodites should grow and later become a hermaphrodite to compete with others for fertilizing eggs (if the substratum will last long enough to allow it to grow large) [2,11]. In fact, dwarf males of the pedunculate barnacle O. warwickii [11] and those of the acorn (sessile) barnacle Chelonibia patula [26] are morphologically indistinguishable from conspecific hermaphrodites (except for smaller size and earlier maturation of the testis), and sexual expression appears to be environmentally determined. Dwarf males of O. warwickii tend to occur on solitary rather than aggregating hermaphrodites [11]. This may suggest that the larvae choose to settle on conspecifics to become dwarf males or on a substratum (crab carapaces) to become hermaphrodites in an adaptive way. Likewise, adaptive sex allocation as a phenotypic response (i.e. more female-biased allocation in small mating groups) is known in an acorn barnacle, Catomerus polymerus [49] (but not in another species, Tetraclita rubescens) [19]. Although fragmentary, these pieces of evidence suggest that sexual systems and sex allocation patterns observed in barnacles are in agreement with sex allocation theories even in species with environmentally determined sexual systems.
Previous theories predict that androdioecy is an extremely rare sexual system compared with hermaphroditism, dioecy or gynodioecy [6,7,50]. In fact, in the best-studied taxon, angiosperms, gynodioecy is much more common than androdioecy [6,50]. However, our results suggest that androdioecy is more common than gynodioecy during the course of evolution from hermaphroditism to dioecy in pedunculate barnacles. To explain this apparent discrepancy between theory and empirical patterns in barnacles, or between patterns observed in flowering plants and barnacles, two questions should be addressed: (i) why androdioecy is common in barnacles when compared with other organisms; and (ii) why barnacles lack gynodioecy. To address the first question, it is important to distinguish two types of androdioecy: one with normal-sized males and the other with dwarf males [7]. For males to evolve in a hermaphroditic population, they must compensate for the disadvantage of lacking female reproductive function. Normal-sized males must attain at least twofold higher mating success than hermaphrodites through male function to have identical fitness [6,7]. Attaining this is difficult since in hermaphroditic populations, male mating success does not increase proportionally to the amount of resource input [2,13]. Thus, most cases of androdioecy with normal-sized males in animals and plants are considered to have evolved from dioecious populations rather than from hermaphroditic populations, by females becoming self-fertile hermaphrodites under low density to ensure fertilization [6,7]. However, dwarf males suffer less from diminishing returns because of their smaller amount of resources, and possibly they have mating advantages over hermaphrodites by being nearer the fertilization site of conspecifcs [1,16]. More importantly, dwarf males need not maintain the twofold higher mating success because of their survival advantage owing to early maturation [15,26]. In fact, a mathematical model showed that smaller males are more likely to evolve than larger males in hermaphroditic barnacles even with a simple trade-off between survival rate and body size (assuming survival rate multiplied by body size is constant) [15]. The conditions for the occurrence of dwarf males would be even more relaxed if the sexual system is environmentally determined, as in this case, the fitness of dwarf males and that of hermaphrodites need not be equal. Although androdioecy with relatively small males has been little known in other taxa outside of thoracican barnacles [7], it may be more common than previously considered.
Concerning the second question (i.e. the lack of gynodioecy in barnacles), it is important to realize that conditions for the evolution of gynodioecy are not a mirror image of conditions for androdioecy [6,50]. When hermaphrodites self-fertilize, for males to evolve, they must attain more than twofold higher reproductive success than male-acting hermaphrodites, whereas females have only to attain less than twofold higher reproductive success. This discrepancy becomes more exaggerated as the degree of selfing and its resultant inbreeding depression increases. Thus, in angiosperms, gynodioecy may evolve relatively easily as a mechanism to avoid inbreeding depression [6,50]. On the other hand, most barnacles do not self-fertilize, and hence require twofold higher reproductive success for females to evolve. Since the optimal allocation of hermaphrodites is female-biased in small mating groups [2], pure females will not have twofold higher reproductive success when compared with hermaphrodites even if they allocate all resources to female function. In short, outcrossing and female-biased sex allocation in hermaphrodites probably prevent gynodioecy from evolving in barnacles.
Most problems in reproductive biology raised by Darwin, such as sexual selection, variation in sex ratios or cross- versus self-fertilization, have been well addressed under the framework of modern evolutionary biology [1–5], but the sexual systems of barnacles recognized by Darwin [8] remain incompletely understood. This study demonstrates the importance of limited mating opportunity and provides an explanation of hermaphroditism, androdioecy and dioecy, as well as the presence of dwarf males, in pedunculate barnacles. However, the forces driving the evolution of sexual systems may differ among taxa. Phylogenetically controlled tests have identified the environmental factors responsible for the evolution of different sexual systems in other taxa, especially plants [41,51]. Few tests exist in animal taxa with diverse sexual systems comparable to those of barnacles [33], and the evolution of such systems in most cases remains to be clarified. Thus, further study is needed on the relative importance of the evolutionary forces producing the diverse sexual systems observed in animals.
Acknowledgements
We thank M. Berggren, Y. Kanoh and S. Ohtsuka for providing samples, M. Ohata and J. Inoue for technical help, K. Wada, W. Newman and M. Grygier for discussion and encouragement, and S. Yamaguchi, K. Sawada, M. Pérez-Losada and the editors and referees for comments on the manuscript. Y.Y. was financed by JSPS KAKENHI 22570020, and J.T.H. was financed by grants from the Danish Agency for Science, Technology and Innovation (FNU) and from the European Union ‘Synthesys’ and ‘Synthesys-2’ programmes.
References
- 1.Ghiselin M. T. 1974. The economy of nature and the evolution of sex. Berkeley, CA: University of California Press [Google Scholar]
- 2.Charnov E. L. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 4.Hardy I. C. W. 2002. Sex ratios: concepts and research methods. Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.West S. A. 2009. Sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 6.Pannell J. R. 2002. The evolution and maintenance of androdioecy. Annu. Rev. Ecol. Syst. 33, 397–425 10.1146/annurev.ecolsys.33.010802.150419 (doi:10.1146/annurev.ecolsys.33.010802.150419) [DOI] [Google Scholar]
- 7.Weeks S. C., Benvenuto C., Reed S. K. 2006. When males and hermaphrodites coexist: a review of androdioecy in animals. Integr. Comp. Biol. 46, 449–464 10.1093/icb/icj048 (doi:10.1093/icb/icj048) [DOI] [PubMed] [Google Scholar]
- 8.Darwin C. 1851. A monograph on the sub-class Cirripedia. I. The Lepadidae. London, UK: Ray Society [Google Scholar]
- 9.Høeg J. T. 1995. Sex and the single cirripede: a phylogenetic perspective. In New frontiers in barnacle evolution (eds Schram R. F., Høeg J. T.), pp. 195–207 Rotterdam, The Netherlands: A. A. Balkema [Google Scholar]
- 10.Vollrath F. 1998. Dwarf males. Trends Ecol. Evol. 13, 159–163 10.1016/S0169-5347(97)01283-4 (doi:10.1016/S0169-5347(97)01283-4) [DOI] [PubMed] [Google Scholar]
- 11.Yusa Y., Takemura M., Miyazaki K., Watanabe T., Yamato S. 2010. Dwarf males of Octolasmis warwickii (Cirripedia: Thoracica): the first example of coexistence of males and hermaphrodites in the suborder Lepadomorpha. Biol. Bull. 218, 259–265 [DOI] [PubMed] [Google Scholar]
- 12.Buhl-Mortensen L., Høeg J. T. 2006. Reproduction and larval development in three scalpellid barnacles, Scalpellum scalpellum (Linnaeus 1767), Ornatoscalpellum stroemii (M. Sars 1859) and Arcoscalpellum michelottianum (Seguenza 1876), Crustacea: Cirripedia: Thoracica): implications for reproduction and dispersal in the deep sea. Mar. Biol. 149, 829–844 10.1007/s00227-006-0263-y (doi:10.1007/s00227-006-0263-y) [DOI] [Google Scholar]
- 13.Charnov E. L. 1987. Sexuality and hermaphroditism in barnacles: a natural selection approach. In Barnacle biology (ed. Southward A. J.), pp. 89–103 Rotterdam, The Netherlands: A. A. Balkema [Google Scholar]
- 14.Yamaguchi S., Yusa Y., Yamato S., Urano S., Takahashi S. 2008. Mating group size and evolutionarily stable pattern of sexuality in barnacles. J. Theor. Biol. 253, 61–73 10.1016/j.jtbi.2008.01.025 (doi:10.1016/j.jtbi.2008.01.025) [DOI] [PubMed] [Google Scholar]
- 15.Urano S., Yamaguchi S., Yamato S., Takahashi S., Yusa Y. 2009. Evolution of dwarf males and a variety of sexual modes in barnacles: an ESS approach. Evol. Ecol. Res. 11, 713–729 [Google Scholar]
- 16.Gotelli N. J., Spivey H. R. 1992. Male parasitism and intrasexual competition in a burrowing barnacle. Oecologia 91, 474–480 10.1007/BF00650319 (doi:10.1007/BF00650319) [DOI] [PubMed] [Google Scholar]
- 17.Ozaki Y., Yusa Y., Yamato S., Imaoka T. 2008. Reproductive ecology of the pedunculate barnacle Scalpellum stearnsii (Cirripedia: Lepadomorpha: Scalpellidae). J. Mar. Biol. Assoc. UK 88, 77–83 10.1017/S0025315408000131 (doi:10.1017/S0025315408000131) [DOI] [Google Scholar]
- 18.Svane I. 1986. Sex determination in Scalpellum scalpellum (Cirripedia: Thoracica: Lepadomorpha), a hermaphroditic goose barnacle with dwarf males. Mar. Biol. 90, 249–253 10.1007/BF00569135 (doi:10.1007/BF00569135) [DOI] [Google Scholar]
- 19.Kelly M. W., Sanford E. 2010. The evolution of mating systems in barnacles. J. Exp. Mar. Biol. Ecol. 392, 37–45 10.1016/j.jembe.2010.04.009 (doi:10.1016/j.jembe.2010.04.009) [DOI] [Google Scholar]
- 20.Newman W. A. 1980. A review of extant Scillaelepas (Cirripedia: Scalpellidae) including recognition of new species from the North Atlantic, western Indian Ocean and New Zealand. Tethys 9, 379–398 [Google Scholar]
- 21.Klepal W. 1987. A review of the comparative anatomy of the males in cirripedes. Oceanogr. Mar. Biol. Annu. Rev. 25, 285–351 [Google Scholar]
- 22.Pagel M. 1997. Inferring evolutionary processes from phylogenies. Zool. Scripta 26, 331–348 10.1111/j.1463-6409.1997.tb00423.x (doi:10.1111/j.1463-6409.1997.tb00423.x) [DOI] [Google Scholar]
- 23.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884 10.1038/44766 (doi:10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Losada M., Høeg J. T., Crandall K. A. 2004. Unraveling the evolutionary radiation of the Thoracican barnacles using molecular and morphological evidence: a comparison of several divergence time estimation approaches. Syst. Biol. 53, 244–264 10.1080/10635150490423458 (doi:10.1080/10635150490423458) [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Losada M., Harp M., Høeg J. T., Achituv Y., Jones D., Watanabe H., Crandall K. A. 2008. The tempo and mode of barnacle evolution. Mol. Phylogenet. Evol. 46, 328–346 10.1016/j.ympev.2007.10.004 (doi:10.1016/j.ympev.2007.10.004) [DOI] [PubMed] [Google Scholar]
- 26.Crisp D. J. 1983. Chelonobia patula (Ranzani), a pointer to the evolution of the complemental male. Mar. Biol. Lett. 4, 281–294 [Google Scholar]
- 27.Gomez E. D. 1975. Sex determination in Balanus (Conopea) galeatus (L.) (Cirripedia Thoracica). Crustaceana 28, 105–107 [Google Scholar]
- 28.McLaughlin P. A., Henry D. P. 1972. Comparative morphology of complemental males in four species of Balanus (Cirripedia Thoracica). Crustaceana 22, 13–30 10.1163/156854072X00642 (doi:10.1163/156854072X00642) [DOI] [Google Scholar]
- 29.Foster B. A. 1980. The marine fauna of New Zealand barnacles (Cirripedia: Thoracica) from New Zealand. N. Z. J. Zool. 7, 523–531 [Google Scholar]
- 30.Zardus J. D., Hadfield M. G. 2004. Larval development and complemental males in Chelonibia testudinaria, a barnacle commensal with sea turtles. J. Crust. Biol. 24, 409–421 10.1651/C-2476 (doi:10.1651/C-2476) [DOI] [Google Scholar]
- 31.Klepal W. 1990. The fundamentals of insemination in cirripedes. Oceanogr. Mar. Biol. Annu. Rev. 28, 353–379 [Google Scholar]
- 32.Pannell J. R. 2002. What is functional androdioecy? Funct. Ecol. 16, 862–865 10.1046/j.1365-2435.2002.06893.x (doi:10.1046/j.1365-2435.2002.06893.x) [DOI] [Google Scholar]
- 33.Weeks S. C., Chapman E. G., Rogers D. C., Senyo D. M., Hoeh W. R. 2009. Evolutionary transitions among dioecy, androdioecy and hermaphroditism in limnadiid clam shrimp (Branchiopoda: Spinicaudata). J. Evol. Biol. 22, 1781–1799 10.1111/j.1420-9101.2009.01813.x (doi:10.1111/j.1420-9101.2009.01813.x) [DOI] [PubMed] [Google Scholar]
- 34.Annandale B. A. 1905. Malaysian barnacles in the Indian Museum, with a list of the Indian Pedunculata. Mem. Asiat. Soc. Beng. 1, 73–81 [Google Scholar]
- 35.Yusa Y., Yamato S., Marumura M. 2001. Ecology of a parasitic barnacle, Koleolepas avis: relationship to the hosts, distribution, left–right asymmetry and reproduction. J. Mar. Biol. Assoc. UK 81, 781–788 10.1017/S0025315401004593 (doi:10.1017/S0025315401004593) [DOI] [Google Scholar]
- 36.Whiting M. F. 2002. Mecoptera is paraphyletic: multiple genes and phylogeny of Mecoptera and Siphonaptera. Zool. Scr. 31, 93–104 10.1046/j.0300-3256.2001.00095.x (doi:10.1046/j.0300-3256.2001.00095.x) [DOI] [Google Scholar]
- 37.Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgens D. G. 1997. The Clustal X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 23, 4876–4882 10.1093/nar/25.24.4876 (doi:10.1093/nar/25.24.4876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronquist F., Huelsenbeck J. P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 10.1093/bioinformatics/btg180 (doi:10.1093/bioinformatics/ btg180) [DOI] [PubMed] [Google Scholar]
- 39.Swofford D. L. 2002. PAUP*: Phylogenetic analysis using parsimony (*and other methods), version 4.0 b10. Sunderland, MA: Sinauer Associates [Google Scholar]
- 40.Posada D., Crandall K. A. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14, 817–818 10.1093/bioinformatics/14.9.817 (doi:10.1093/bioinformatics/14.9.817) [DOI] [PubMed] [Google Scholar]
- 41.Crawford M., Jesson L. K., Garnock-Jones P. J. 2009. Correlated evolution of sexual system and life-history traits in mosses. Evolution 63, 1129–1142 10.1111/j.1558-5646.2009.00615.x (doi:10.1111/j.1558-5646.2009.00615.x) [DOI] [PubMed] [Google Scholar]
- 42.Organ C. L., Janes D. E., Meade A., Pagel M. 2009. Genotypic sex determination enabled adaptive radiations of extinct marine reptiles. Nature 461, 389–392 10.1038/nature08350 (doi:10.1038/nature08350) [DOI] [PubMed] [Google Scholar]
- 43.Pagel M., Meade A. 2006. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. Am. Nat. 167, 808–825 10.1086/503444 (doi:10.1086/503444) [DOI] [PubMed] [Google Scholar]
- 44.Maddison W. P., Maddison D. R. 2009. Mesquite: a modular system for evolutionary analysis, version 2.71. See http://mesquiteproject.org
- 45.Eppley S. M., Jesson L. K. 2008. Moving to mate: the evolution of separate and combined sexes in multicellular organisms. J. Evol. Biol. 21, 727–736 10.1111/j.1420-9101.2008.01524.x (doi:10.1111/j.1420-9101.2008.01524.x) [DOI] [PubMed] [Google Scholar]
- 46.Høeg J. T., Pérez-Losada M., Glenner H., Kolbasov G. A., Crandall K. A. 2009. Evolution of morphology, ontogeny and life cycles within the Crustacea Thecostraca. Arthropod Syst. Phylogeny 67, 199–217 [Google Scholar]
- 47.Barnes M. 1989. Egg production in cirripedes. Oceanogr. Mar. Biol. Annu. Rev. 27, 91–166 [Google Scholar]
- 48.Walker G. 1995. Larval settlement: historical and future perspectives. In New frontiers in barnacle evolution (eds Schram R. F., Høeg J. T.), pp. 69–85 Rotterdam, The Netherlands: A. A. Balkema [Google Scholar]
- 49.Raimondi P. T., Martin J. E. 1991. Evidence that mating group size affects allocation of reproductive resources in a simultaneous hermaphrodite. Am. Nat. 138, 1206–1217 10.1086/285278 (doi:10.1086/285278) [DOI] [Google Scholar]
- 50.Charlesworth B., Charlesworth D. 1978. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997 10.1086/283342 (doi:10.1086/283342) [DOI] [Google Scholar]
- 51.Torices R., Anderberg A. A. 2009. Phylogenetic analysis of sexual systems in Inuleae (Asteraceae). Am. J. Bot. 96, 1011–1019 10.3732/ajb.0800231 (doi:10.3732/ajb.0800231) [DOI] [PubMed] [Google Scholar]