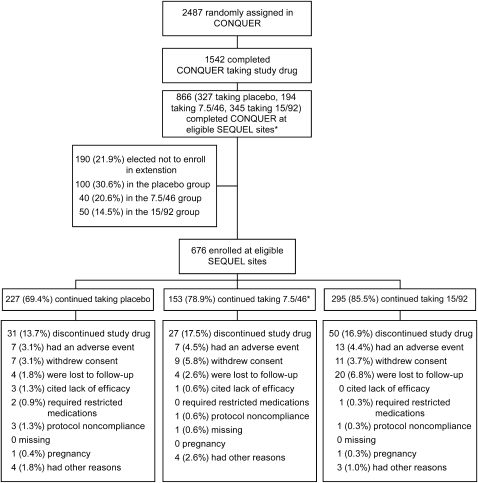
FIGURE 1.
Trial profile. Standardized lifestyle intervention was used across all treatment groups. Of the 27 subjects who discontinued the study drug because of adverse events during the SEQUEL study, the event began during the CONQUER study for 3 subjects (one in each treatment group). For the remaining 24 subjects, the adverse event leading to discontinuation of the study drug began during the SEQUEL study. *One subject in the 7.5/46 group enrolled in the study but discontinued before receiving the study drug. 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; 15/92, 15 mg phentermine/92 mg controlled-release topiramate.