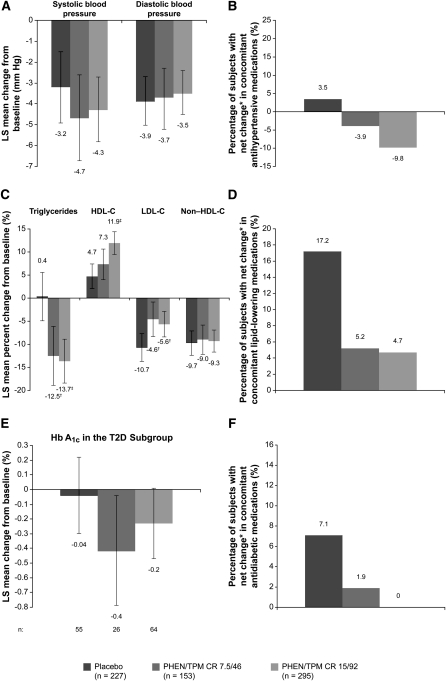
FIGURE 4.
Effects of PHEN/TPM CR on cardiometabolic variables. LS mean changes (95% CI) in (A) blood pressure, (B) antihypertensive medications, (C) lipid variables, (D) lipid-lowering medications, (E) Hb A1c, and (F) antidiabetic medications from baseline (week 0) to week 108 (ITT-LOCF). Changes in Hb A1c represent the T2D subgroup. Changes in concomitant medications represent the safety study. Standardized lifestyle intervention was used across all treatment groups. *Percentage increase minus percentage decrease; P < 0.05 for between-group differences. †P < 0.01 compared with placebo; ‡P < 0.0001 compared with placebo. Hb A1c, glycated hemoglobin; HDL-C, HDL cholesterol; ITT, intent-to-treat; LDL-C, LDL cholesterol; LOCF, last observation carried forward; LS, least-squares; PHEN/TPM CR 7.5/46, 7.5 mg phentermine/46 mg controlled-release topiramate; PHEN/TPM CR 15/92, 15 mg phentermine/92 mg controlled-release topiramate; T2D, type 2 diabetes.