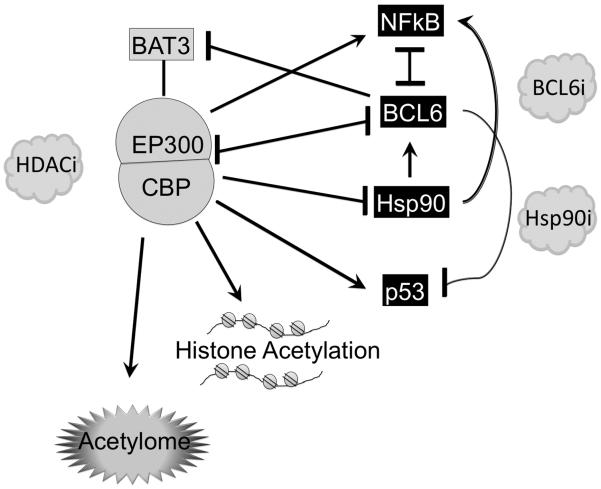
Figure 2. Disruption of CBP and p300 acetyltransferases in B-cell lymphomas.
CBP and p300 have both overlapping and unique functions. p300 acts as a co-activator for NFkB, and activates p53 and on the other hand can attenuate Hsp90 chaperone functions and BCL6 transcriptional repressor functions. P300 and CBP also acetylate histones and potentially hundreds of other proteins in the acetylome. Deacetylated Hsp90 maintains BCL6 expression, which in turn suppresses p300 and its essential cofactor BAT3 and also represses p53. This vicious circle can be disrupted by treating lymphoma cells with BCL6 inhibitors, Hsp90 inhibitors or HDAC inhibitors, and these agents are synergistic when administered in combination. Blockade of p300 rescues DLBCL cells from being killed by BCL6 inhibitors and HDAC inhibitors. CBP also acetylates p53 and BCL6. Somatic heterozygous mutations or deletions of the CREBBP locus occur in 41.5% of GCB-DLBCLs, 12% of ABC-DLBCLs, and 32.6% of FL cases. EP300 mutations occur in 10% of DLBCLs. All cases seem to have in common disruption of the histone acetyltransferase catalytic domain, and the resulting truncated or mutant proteins may have dominant negative or gain of function properties, or may simply result in a reduced dosage of histone acetyltransferases. These effects would presumably lock DLBCL cells in a state with inactivated p53, and activated BCL6 and Hsp90, in addition to many other possible downstream effects.