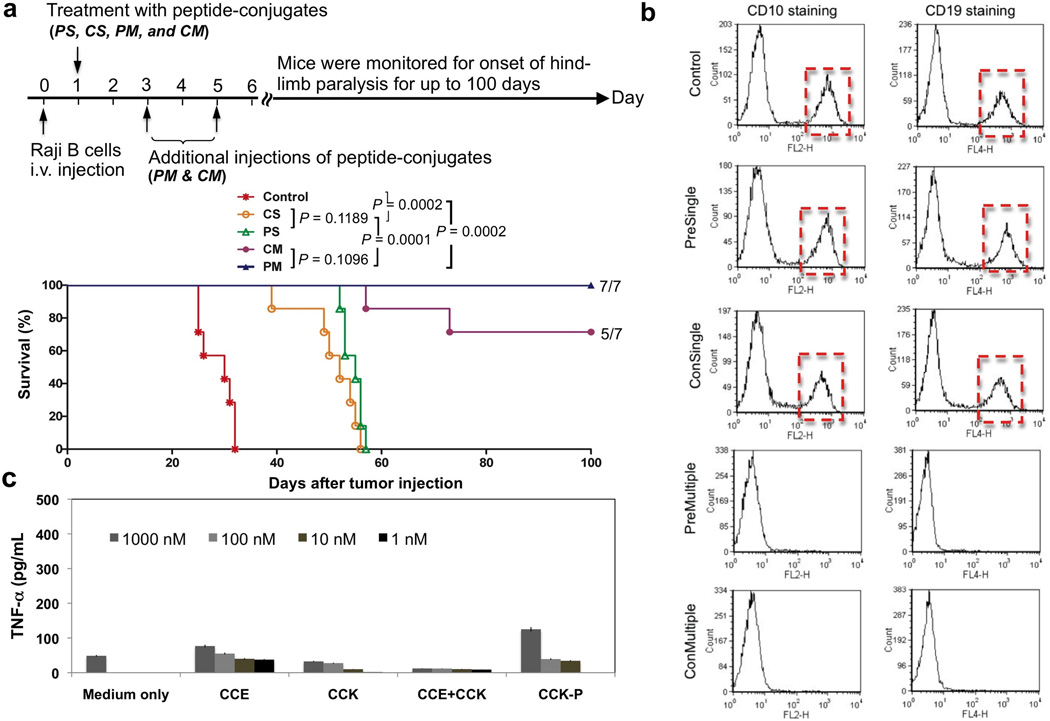
Figure 3.
Therapeutic efficacy of drug-free macromolecular therapeutics against systemically disseminated Raji B cell lymphoma in C.B.-17 SCID mice (7 mice per group). (a) Top panel shows timeline for the in vivo efficacy study. Four million Raji B cells were injected into the tail vein on day 0 to initiate the disseminated disease. The incidence of hind-limb paralysis or survival of mice was monitored until day 100. Five groups of animals were evaluated: untreated controls; consecutive administration of single dose (CS); premixed administration of single dose (PS); consecutive administration of three doses at days 1, 3, and 5 (CM); and v) premixed administration of three doses at days 1, 3, and 5 (PM). Consecutive administration involved the i.v. injection of 50 µg/20 g Fab’-CCE first and 1 h later the i.v. administration of 324 µg/20 g CCK-P conjugate; For premixed administration, the two conjugates were mixed together 1 h before injection via the tail vein. Bottom panel shows survival rate of tumor-bearing mice that received above treatments. The curve was presented in a Kaplan-Meier plot with indication of numbers of long-term survivors (7 mice per group); (b) Estimation of residual Raji B lymphoma cells in the bone marrow. Shown are results from representative mice that received the indicated treatment. Revealed are histograms of bone marrow cells isolated from mice (as indicated) followed by staining with PE mouse anti-human CD10 and APC mouse anti-human CD19. (c) Preliminary evaluation of immunogenicity. TNF-α released from RAW 264.7 cells upon exposure to peptides (1 day) and HPMA copolymer-peptide conjugate (7 days) in vitro. The values are shown as averages (n = 4) ± S.D.