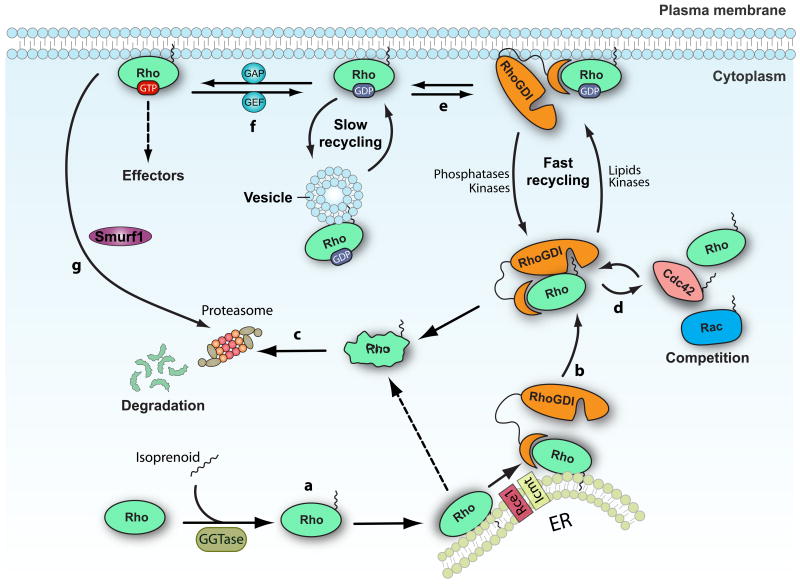
Figure 1. The RHOGDI cycle.
(a) Newly synthesized RHO family GTPases are geranylgeranylated and then post-translationally modified by the protease Ras-converting enzyme 1 (RCE1) and by isoprenylcysteine carboxyl methyltransferase (ICMT) at the cytoplasmic face of the endoplasmic reticulum (ER). (b) After geranylgeranylation, RHO proteins associate with RHO specific guanine nucleotide dissociation inhibitors (RHOGDIs), which sequester them in the cytosol and protect them from degradation. (c) Free prenylated cytosolic RHO GTPases are unstable and are rapidly degraded by the proteasome. (d) Several RHO GTPases can associate with RHOGDI and compete for its binding. Overexpression of a GTPase can displace the endogenous RHO proteins from RHOGDI targeting them for degradation. (e) The rate of cycling of the RHOGDI -RHO GTPase complex between the cytosol and the membrane can be regulated by post-translational modifications on both the RHO GTPases and the RHOGDI, which modulate the affinity of the interaction. A slower pathway for recycling RHO proteins through vesicle trafficking has also been postulated. (f) Once at the membrane, the RHO GTPases can be activated by guanine nucleotide exchange factors (GEFs) and bind to downstream effectors. Following inactivation by GTPase-activating proteins (GAPs), RHO GTPases are extracted from the membrane by RHOGDI. (g) Active RHOA can also be targeted for degradation by the ubiquitin ligase SMAD ubiquitylation regulatory factor 1 (Smurf1). GGTase, geranylgeranyl transferase.