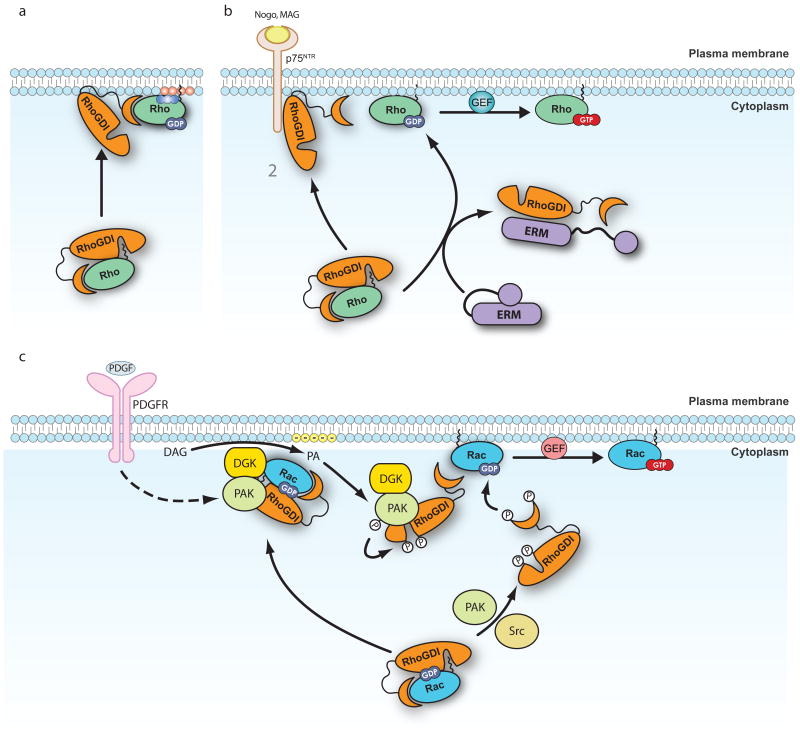
Figure 3. Mechanisms of regulation of the RHOGDI-RHOGTPase interaction.
a | Release by lipids: The presence of acidic phospholipids can promote the release of RHOGTPases from RHO-specific guanine nucleotide dissociation inhibitors (RHOGDIs). Phospholipids mediate a partial opening of the complex that exposes the GTPases to RHO-specific guanine nucleotide exchange factors (RHOGEFs) or other dissociation factors such as the ones described in b. b | Release by protein–protein interactions: p75 neurotrophin receptor (p75NTR) and ezrin, radixin and moesin (ERM) proteins interact with RHOGDIs and facilitate the release of RHOA, which can then be activated by specific RHOGEFs. The interaction of p75NTR with RHOGDIs is enhanced by myelin derived proteins such as myelin-associated glycoprotein (MAG) and neurite outgrowth inhibitor (NOGO). ERM proteins also need to be activated to interact with RHOGDI. c | Release by phosphorylation. Phosphorylation of RHOGDI Ser, Thr and Tyr residues promote the release of RHO GTPases. Depending on the residues phosphorylated, this release can be specific for a single RHO GTPase, or affect multiple RHO GTPases simultaneously (Table 1). For example p21-activated kinase (PAK)- or FER-mediated phosphorylation promote the specific release of RAC1, but not RHOA or CDC42. Some kinases act in concert to target the RAC1–RHODI complex to the membrane and regulate the local release of RAC1 at specific site on the membrane, where it is subsequently activated by RHOGEFs. Diacylglycerol kinase-ζ (DGKζ) forms a complex with PAK, RAC1 and RHOGDI. In response to platelet derived growth factor (PDGF), DGKζ stimulates the production of phosphatidic acid (PA), which induces PAK activity. Active PAK then phosphorylates RHOGDI and releases RAC1 for activation. Positively charged phospholipids are indicated in blue, negatively charged phopholipids in red. DAG, diacylglycerol; PDGFR, PDGF receptor.