Abstract
Transcranial magnetic stimulation (TMS) has developed into a powerful tool for studying human brain physiology and brain–behavior relations. When applied in sessions of repeated stimulation, TMS can lead to changes in neuronal activity/excitability that outlast the stimulation itself. Such aftereffects are at the heart of the offline TMS protocols in cognitive neuroscience and neurotherapeutics. However, whether these aftereffects are of applied interest critically depends on their magnitude and duration, which should fall within an experimentally or clinically useful range without increasing risks and adverse effects. In this short review, we survey combined TMS-EEG studies to characterize the TMS-aftereffects as revealed by EEG to contribute to the characterization of the most effective and promising repetitive TMS-parameters. With one session of conventional repetitive TMS (of fixed pulse frequency), aftereffects were consistently comparable in magnitude to EEG-changes reported after learning or with fatigue, and were short-lived (<70 min). The few studies using recently developed protocols (such as theta burst stimulation) suggest comparable effect-size but longer effect-durations. Based on the reviewed data, it is expected that TMS-efficacy can be further promoted by repeating TMS-sessions, by using EEG-gated TMS to tailor TMS to current neuronal state, or by other, non-conventional TMS-protocols. Newly emerging developments in offline TMS research for cognitive neuroscience and neurotherapeutics are outlined.
Keywords: Transcranial magnetic stimulation, Electroencephalography, Aftereffects, Repetitive TMS, Safety
Introduction
Transcranial magnetic stimulation (TMS) is increasingly used for noninvasive stimulation of the human brain in healthy participants and patients to study brain–behavior relations, the pathophysiology of diseases, and the potential of neuromodulation for rehabilitation and therapy (e.g. Walsh and Cowey 2000; Kobayashi and Pascual-Leone 2003; Rossi and Rossini 2004; Ridding and Rothwell 2007; Fregni and Pascual-Leone 2007; Hallett 2007). TMS can be applied as one stimulus at a time (single pulse), as trains of stimuli delivered at a fixed frequency (conventional repetitive TMS, usually in the range of 1–20 Hz), or in more complex trains combining different frequencies, such as for example 50 Hz pulse-trains repeated at a rate of 5 Hz, termed theta burst stimulation (TBS) (Huang et al. 2005). In repeated paired associative stimulation (PAS) (Classen et al. 2004), repetitive median nerve stimulation at very low frequencies (e.g. 0.1 Hz) is combined with concurrent contralateral post-rolandic TMS. Provided safety guidelines are met (Wassermann 1998; Rossi et al. 2009), TMS is considered safe in humans as shown through comprehensive surveys of potential adverse effects and complications (Chen et al. 1997; Wassermann 1998; Machii et al. 2006; Bae et al. 2007; Rossi et al. 2009).
An important aspect of TMS is that the effects of each single pulse or single-train can summate with repeated application, leading to effects outlasting a stimulation session (Robertson et al. 2003; Rossi and Rossini 2004; Ridding and Rothwell 2007; Hallett 2007). This has been termed the offline repetitive TMS (rTMS) approach, which is used to transiently modulate brain function in healthy participants beyond TMS to probe for the implication of the stimulated area (or network) in perception and cognition (e.g. Robertson et al. 2003), or with the aim of modifying brain activity over longer time-scales in patients with specific neurological disorders for therapeutic intent (e.g. Kobayashi and Pascual-Leone 2003; Rossi and Rossini 2004; Fregni and Pascual-Leone 2007; Hallett 2007). However, while many studies have used these protocols with the above-mentioned aims, little is known on the magnitude and duration of the induced changes broken down according to the parameter space of offline rTMS-protocols. Because TMS can transiently induce as well as interfere with electrical brain activity (reviews in Komssi and Kähkönen 2006; Miniussi and Thut 2009; Taylor et al. 2008), surveying combined TMS-EEG studies for TMS-induced aftereffects can provide useful information here. In particular, EEG-activity is likely to be a more sensitive measure for evaluating TMS-impact on brain function than behavioral effects, given that on several instances robust TMS-aftereffects in EEG-activity have been reported without a behavioral correlate, when both EEG and behavior have been co-examined (e.g. Rossi et al. 2000; Hansenne et al. 2004; Holler et al. 2006; Ortu et al. 2009). In analogy, TMS-aftereffects in electromyographic (EMG) measures of motor cortex excitability have been observed in the absence of apparent changes in amplitude or velocity of voluntary movements (e.g. Muellbacher et al. 2000). While it is sensible to base the design of TMS experiments on observable behavioral manifestations, it is therefore also of interest to interrogate potentially more sensitive physiological changes to assess the impact of TMS.
This review focuses on TMS-aftereffects on EEG-activity in repeated TMS designs. In surveying combined TMS-EEG research, we shall address the following questions: (1) Which rTMS parameters evoke the most reliable aftereffects in terms of magnitude and direction of changes in EEG/evoked potentials (EPs)? Is the effect-size in the order of physiological changes, such as those observed after learning or fatigue, or does it markedly exceed the normal range? (2) Which rTMS parameters do evoke the most long-lasting effect? The results are discussed in terms of future developments for boosting the efficacy and utility of rTMS protocols.
Methods
Literature Review
Using Pubmed and the references from relevant articles, we identified more than 100 TMS-EEG studies in >1000 participants that have been published over the last 20 years (Jan 1989–May 2009). Inclusion criterion for review was quantification of aftereffects in EEG activity/Evoked Potentials (EPs) after rTMS delivery.
We reviewed all reports and noted (i) article references, (ii) total number of participants and population (i.e. healthy or patients), (iii) TMS parameters (including number of trains, train-duration, intertrain interval, stimulation site and intensity, number of applied pulses), (iv) EEG-measures, (v) timing of EEG-recordings relative to TMS (before, during or after), (vi) presence or absence of aftereffects (and their nature), (vii) aftereffect size (percent change relative to pre-TMS or sham control) and (viii) duration of aftereffects on EEG (when assessed). See Table 2.
Table 2.
Quantified EEG changes after repeated TMS pulses
| Authors | Subjects | TMS-parameters | EEG-measures | Timing of EEG | Aftereffects | Duration of aftereffects |
|---|---|---|---|---|---|---|
| Urushihara et al. (2006) | N = 9 healthy | 0.2 Hz/21 min of monophasic M1,PM,SMA/85%rMT/250p | SEP-amplitude | Before and after TMS | Yes: ~55% facilitation after PM-stim | Not assessed |
| Hosono et al. (2008) | N = 13 healthy | 0.2 Hz/20 min/mono- vs. biphasic PM,sham/85%rMT/250p | SEP-amplitude | Before and after TMS | Yes: ~30–40% facilitation for mono-but no effect for bi-phasic | At least 10 min but recovered after 30 min |
| Van Der Werf and Paus (2006) | N = 12 | 0.6 Hz/15 min M1,PM/90%rMT/540p | TMS-induced response | Before and after TMS | Yes: ~60% amplitude decrease, reduction in induced activity (theta) | At least 40 min not studied longer |
| Satow et al. (2003) | N = 8 healthy | 0.9 Hz/16.6 min S1/90%rMT/900p | SEP-amplitude | Before and after rTMS | No | n.a. |
| Rollnik et al. (2004) | N = 11 healty | 0.9 Hz/15–20 × 60 s trains frontal/90%MT | Error-related negativity | After TMS | Yes: attenuation of ERN | At least 3 min not studied longer |
| Chen et al. (2003) | N = 8 healthy | 0.9 Hz/15 min PM/90%aMT/818p | Movement-related power/coherence (task) | Before and after TMS | Yes: less task-related alpha/beta decrease coherence increase (alpha) | Up to 15 min recovered after 30 min |
| Enomoto et al. (2001) | N = 7 healthy | 1 Hz/3.3 min PM, M1,S1/110%aMT/200p | SEP-amplitude | Before and after rTMS | Yes: ~40% suppression with M1 but not PM or S1 | Up to 60 min recovered after 70 min |
| Restuccia et al. (2007) | N = 8 healthy | 1 Hz/20 min S1/sham/80%MT/1200p | Early/late HF-SEPs | Before and after TMS | Yes: ~18/26% facilit./suppress. after S1 relative to sham | Up to 15 min recovered after 20 min |
| Bohotin et al. (2002) | N = 24 healthy | 1 Hz/15 min occipital/100%PT/900p | VEP-amplitude & habituation | Before and after TMS | Yes: ~15% suppression in controls | Up to 15–30 min |
| Fumal et al. (2003) | N = 24 healthy | 1 Hz/15 min occipital/100%PT/900p | VEP-amplitude & habituation | Before and after TMS | Yes: ~15% suppression | Up to 33 min recovery after 40 min |
| Schutter and van Honk (2003) | N = 8 healthy | 1 Hz/20 min occipital/110%MT/1200p | VEP-amplitude | After TMS and sham | Yes: ~55% suppression | Not assessed |
| Thut et al. (2003) | N = 6 healthy | 1 Hz/10 min Occipital/110%PT/600p | VEP-amplitude alpha-changes | Before and after TMS | Yes: ~40% suppression of desynchronization (alpha) | At least 8 min recovered after 20 min |
| Evers et al. (2001) | N = 14 healthy | 1 Hz/2 min DLPFC/95%MT/120p | Visual P300 | Before and after TMS | No | n.a. |
| Hansenne et al. (2004) | N = 17 healthy | 1 Hz/10 and 15 min DLPFC/100%MT/600–900p | Auditory P300 | Before and after TMS | Yes/No: latency increase after 15 min but not 10 min rTMS | Not assessed |
| Rossi et al. (2000) | N = 5 healthy | 1 Hz/15 min M1/110%MT/900p | Readiness potential | After TMS and Sham | Yes: ~30% focal amplitude decrease | Not assessed |
| Strens et al. (2002) | N = 15 healthy | 1 Hz/25 min M1/90%aMT/1500p | Spectral power coherence (rest and task) | Before and after TMS | Yes: ~15% focal increase in coherence (alpha) | Up to 25 min recovered after 30 min |
| Tamura et al. (2005) | N = 12 healthy | 1 Hz/10 min M1/95%rMT/600p | Movement-related oscillations | Control and after TMS | Yes: ~10% decrease in synchronization (beta) | Not assessed |
| Brignani et al. (2008) | N = 6 healthy | 1 Hz/10 min M1/110%MT/600p | EEG power (rest) | During TMS | Yes: max 82% focal, cumulative increase in power (alpha) | Not assessed |
| Pastor et al. (2006) | N = 6 healthy | 1 Hz/10 min Cerebellum/60%max/600p | Steady-state potentials | Before and after TMS | Yes: change in prefrontal oscillations (gamma) | At least 7.5 min not studied longer |
| Schutter et al. (2001) | N = 12 healthy | 1 Hz/20 min DLPFC/130%MT/1200p | Spectral power (rest) | After TMS and sham | Yes: increase in prefrontal power (theta) | At least 1 h not studied longer |
| Ragert et al. (2004) | N = 13 healthy | 5 Hz/5 × 5 × 10 s: 5 s ITI S1/90%MT1250p | Paired pulse SEP-inhibition | Before and after TMS | Yes: suppression of inhibition with ipsil-vs. contralateral S1 | At least 1h not studied longer |
| Holler et al. (2006) | N = 8 healthy | 5 Hz/5 × 60 s train: 60 s ITI M1/90%rMT/1500p | Anticipatory CNV | Before and after TMS/sham | Yes: focal amplitude increase | Not assessed |
| Oliviero et al. (2003) | N = 16 healthy | 5 Hz/10 s single train M1/100%aMT/50p | Spectral power coherence (rest and task) | Before and after TMS | Yes: ~25% focal coherence decrease (alpha) | Immediately after TMS recovered after 25 min |
| Fuggetta et al. (2008) | N = 11 healthy | 5 Hz/20 × 4 s trains: 30 s ITI M1/80–100%MT/400p | EEG power and coherence (rest) | During TMS | Yes: ~30–40% focal incr/decr in alpha and beta power/coherence | Not assessed |
| Huber et al. (2007) | N = 10 healthy | 5 Hz/5 × 6 × 10 s train: 5 s ITI M1/90%rMT/1500p | Sleep EEG | After TMS and sham | Yes: ~40% local increase in slow wave activity | Up to 30 min of nREM recovered after 31 min |
| Esser et al. (2006) | N = 7 healthy | 5 Hz/5 × 6 × 10 s train: 5 s ITI M1/90%rMT/1500p | TMS-induced response | Before and after rTMS | Yes: ~60% amplitude increase | At least 10 min not studied longer |
| Restuccia et al. (2007) | N = 8 healthy | 10 Hz/12 × 10 s trains: 20 s ITI S1/sham/80%MT/1200p | Early/late HF-SEPs | Before and after TMS | Yes: ~–/14% –/facilit. after S1 relative to sham | At least 20 min |
| Bohotin et al. (2002) | N = 30 Migraine | 10 Hz/18 × 5 s trains: 10 s ITI occipital/100%PT/900p | VEP-amplitude & habituation | Before and after TMS | Yes: ~15% facilitation in migraineurs | Up to 9–30 min |
| Jing et al. (2001b) | N = 15 healthy | 10 Hz/2 × 3 s train: 5 min ITI DLPFC/100%MT/60p | Auditory P300 | Before and after TMS | Yes: ~15–25% decrease in N100-amplitude, changes in P200/P300-latencies | Not assessed |
| Jing et al. (2001a) | N = 29 healthy | 10 Hz/2 × 3 s train: 5 min ITI DLPFC/100%MT/60p | Auditory P300 | Before and after TMS | Yes: latency-increase | Not assessed |
| Griskova et al. (2007) | N = 18 healthy | 10 Hz/100 × 2 s train: 10 s ITI DLPFC/110%MT/2000p | Spectral power (rest) | Before and after TMS | Yes: ~400% increase in delta-power | At least 10 min, not studied longer |
| Okamura et al. (2001) | N = 32 healthy | 10 Hz/2 × 3 s train: 300 s ITI Frontal/100%MT/60p | Spectral power (rest) | Before and after TMS | Yes: change in peak frequency and power (~10%, many frequencies) | At least 5 min not studied longer |
| Jing and Takigawa (2000) | N = 19 healthy | 10 Hz/2 × 3 s train: 5 min ITI DLPFC/100%MT/60p | Coherence (rest) | Before and after TMS | Yes: max 32% focal directed coherence increase (alpha) | At least 5 min not studied longer |
| Klimesch et al. (2003) | N = 16 healthy | High frequency/IAF-20 Hz 1.2–4.8 s trains: >12 s ITI parietal,frontal/110%MT/24p | Band power and event-related desynchronization | Before and after TMS | Yes: ~40% increase of desynchronization (alpha) | Not assessed |
| Evers et al. (2001) | N = 14 healthy | 20 Hz/3 × 5 s train: 1 min ITI DLPFC/95%MT/300p | Visual P300 | Before and after TMS | Yes: latency-decrease | Not assessed |
| Graf et al. (2001) | N = 8 healthy | 20 Hz/40 × 2 s train: 28 s ITI DLPFC/90%MT/1600p | Sleep EEG | Before and after TMS | Yes: minor reduction in sleep stage 1 | Not assessed |
| Cohrs et al. (1998) | N = 13 healthy | 20 Hz/160 × 0.25 s: 8 s ITI DLPFC/120%rMT/800p | Sleep EEG | After TMS and sham | Yes: delay in REM-latency prolonged nREM-REM cycle | Not assessed |
| Schutter et al. (2003) | N = 5 healthy | 25 Hz/80 × 10 s train: 5 s ITI Cerebellum/80%MT/2000p | Spectral power (rest) | Before and after TMS | Yes: change in prefrontal asymmetry (gamma) | At least 15 min not studied longer |
| Ishikawa et al. (2007) | N = 12 healthy | cThetaBS/40 s M1,S1/80%aMT/600p | SEP-amplitude | Before and after TMS | Yes: ~20% suppression with S1 vs. ~50% facilitation with M1 | Up to 53 min recovered after 60 min |
| Sağlam et al. (2008) | N = 8 healthy | cThetaBS/40 s M1,S1/80%aMT/600p | EEG-EMG-coherence | Before and after TMS | Yes: ~50% suppression with M1 but not S1 | Up to 30–60 min recovered after 90 min |
| Schindler et al. (2008) | N = 4 healthy | cThetaBS/33 s right FEF/80%rMT/600p | Spectral analysis (rest) | Before and after TMS | Yes: ~enhanced synchronization in stimulated hemisphere | At least 60 min not studied longer |
| Ortu et al. (2009) | N = 7 healthy | cThetaBS/40 s M1,PM/80%aMT/600p | Readiness potential | Before and after TMS | Yes: ~30–50% suppression with left M1 but nor right M1 nor PM | At least 30 min not studied longer |
| Grossheinrich et al. (2009) | N = 12 healthy | cThetaBS/40 s DLPFC,mPFC/80%rMT/600p imThetaBS as sham | Spectral analysis (rest) | Before and after TMS | No | n.a. |
| iThetaBS/20 × 2 s: 8 s ITI imThetaBS as sham | Spectral analysis | Before and after TMS | Yes: alpha-power enhancement after DLPFC | Up to 50 min not studied longer | ||
| Poreisz et al. (2008) | N = 12 healthy | cThetaBS/40 s S1,Sham/80%aMT/600p | Laser-evoked potential | Before and after TMS | Yes: ~15% suppression of N2 | Up to 8–10 min not studied longer |
| iThetaBS/20 × 2 s: 8 s ITI S1,Sham/80%aMT/600p | Laser-evoked potential | Before and after TMS | Yes: ~20% suppression of N2 | Up to 8–10 min not studied longer | ||
| imThetaBS/8 × 5 s: 10 s ITI S1,Sham/80%aMT/600p | Laser-evoked potential | Before and after TMS | Yes: ~25% suppression of N2 | Up to 8–10 min not studied longer | ||
| Katayama and Rothwell (2007) | N = 11 healthy | iThetaBS/19 × 2 s: 8 s ITI M1,S1/80%aMT/600p | SEP-amplitude | Before and after TMS | Yes: ~10–20% facilitation with S1 but not M1 | Up to 30 min not studied longer |
| Tsuji and Rothwell (2002) | N = 9 healthy | PAS, 30 min M1/105%rMT/180p | SEP-amplitude | Before and after TMS | Yes: ~40–60% facilitation | Up to 10 min recovered after 20 min |
| Wolters et al. (2005) | N = 64 healthy | PAS, 30 min M1,S1/150%rMT/180p | SEP-amplitude | Before and after TMS | Yes: ~15–20% facilitation with S1 but not M1 | Up to 30 min recovered after 60 min |
| Huber et al. (2008) | N = 19 healthy | PAS, 22.5 min M1/130%rMT/90p | TMS-induced response | Before and after TMS | Yes: ~10% facilitation or 10% inhibition depending on ISI | At least 2 min not studied longer |
| Slow wave activity (sleep) | After TMS | Yes: correlation with change in TMS-induced response | At least 90 min not studied longer |
Studies ordered according to protocols and EEG-measure
TMS-parameters: iThetaBS: intermittent Theta Burst Stimulation; cThetaBS: continuous Theta Burst Stimulation, PAS: repeated paired associative stimulation; ITI: inter-train interval; aMT: active motor threshold; rMT: resting motor threshold; PT: phosphene threshold; p: pulses; IAF: individual alpha frequency; Stimulation sites DLPFC: dorsolateral prefrontal cortex, PM: premotor; EEG-measures SEP: somatosensory evoked potential; HF-SEP: high-frequency SEPs
Review-Sample
The reviewed study sample (n = 51 experiments in ~650 participants with the majority being healthy volunteers) represents approximately 40% of all TMS-EEG studies identified during the reviewed period.
The reminder of the TMS-EEG studies (other 60%) consisted of single-pulse TMS experiments studying the TMS-evoked electrical activity and its spreading immediately after stimulation (<1 s) in the absence of pulse-effect summation, or of rTMS experiments that inspected background EEG for TMS-induced epileptiform activity for safety evaluation (for a detailed review of this aspect see Rossi et al. 2009).
All reviewed studies either used TMS protocols with repeated pulse administration at fixed pulse repetition frequencies (conventional rTMS protocols) or the protocols of TBS (Huang et al. 2005) or PAS (Classen et al. 2004). Within the conventional protocols, only a discrete number of frequencies were tested (0.9–1 Hz: n = 17, 5 Hz: n = 6, 10 Hz: n = 8, 20 Hz: n = 4, 25 Hz: n = 1) often in association with varying number of trains, train-duration, intertrain interval, and TMS-intensities across studies, leading to a large number of employed parameter-combinations (see tables). Three studies used very low frequencies of 0.6 Hz (Van Der Werf and Paus 2006) and 0.2 Hz (Urushihara et al. 2006; Hosono et al. 2008) and are discussed separately where of interest. To reduce this parameters space, we collapse for our analysis across low-frequency (0.9–1 Hz) versus high-frequency (5 Hz and above) protocols. This grouping seems reasonable also because previous studies on motor cortex excitability have reported these two protocols to differ in terms of aftereffect-direction (suppression vs. facilitation) (e.g. reviewed in Hallett 2007). Note that no TMS-EEG study has looked at aftereffects of conventional TMS in the frequency-range between 1 and 5 Hz, where the reversal of effect-direction is to be expected. In regards to TBS and PAS, employed parameters were more uniform across studies.
Table 1 provides a summary of TMS parameters broken down by TMS-protocols (average and range over studies/experiments). The figures and Table 2 depict the results per individual study/experiment.
Table 1.
TMS parameters, aftereffect-size and aftereffect-duration broken down according to TMS protocols
| Protocol | Total N subjects |
Mean total N of pulse |
Mean N of pulses per train |
Mean train-duration |
Mean N of trains |
Mean intertrain interval |
Mean intensity | Mean effect-size | Mean effect-duration (time until recovery) |
|---|---|---|---|---|---|---|---|---|---|
| Conventional Low-F: 0.9–1 Hz | N = 177 | n = 882 (200–1500) | n = 882 (200–1500) | 15 min (3.3–25 min) | n = 1 | – | 101%MT (80–130%MT) | 31% (n = 11: 10–82%) | 31 min (n = 8: 15–70 min) |
| Conventional High-F: 5–25 Hz | N = 251 | n = 704 (24–2000) | n = 60 (20–300) | 9 s (2–60 s) | n = 17 (1–100) | 102 s (5–300 s) | 96%MT (80–110%MT) | 30% (n = 11: 10–60%) | 28 min (n = 3: 25–30 min) |
| cTBS | N = 55 | n = 600 (600–600) | n = 600 (600–600) | 39 s (33–40 s) | n = 1 | – | 80%MT (80–80%MT) | 35% (n = 5: 15–50%) | 70 min (n = 3: 60–90 min) |
| iTBS | N = 35 | n = 600 (600–600) | n = 30.3 (30–31) | 2 s (2–2 s) | n = 19.5 (19–20) | 8 s (8–8 s) | 80%MT (80–80%MT) | 17.5% (n = 2: 15–20%) | no data |
| PAS | N = 92 | n = 150 (90–180) | – | – | – | – | 128%MT (105–150%MT) | 22.5% (n = 3: 10–50%) | 40 min (n = 2: 20–60 min) |
Values represent means or ranges (minimum–maximum)
Results
TMS-Induced Aftereffects in EEG: Affected EEG/EP-Measures
Aftereffects were observed on a variety of measures including somatosensory, visual, cognitive and motor potentials as well as oscillatory activity (Table 2). In general, TMS affected a specific component of the potentials depending on site of stimulation, e.g. C1 after occipital pole stimulation (Schutter and van Honk 2003), distinct SEP-components after stimulation of sensory/motor cortices (e.g. Katayama and Rothwell 2007; Ishikawa et al. 2007; Restuccia et al. 2007), P300 after prefrontal stimulation (e.g. Evers et al. 2001; Hansenne et al. 2004; Jing et al. 2001a), or the negative slope of the motor readiness potential after M1-stimulation (Rossi et al. 2000; Ortu et al. 2009). With respect to oscillatory activity, rolandic alpha- and beta-oscillations were frequently affected after sensory or motor cortex stimulation, both regarding movement-related changes (e.g. Strens et al. 2002; Tamura et al. 2005) as well as background activity at rest (Fuggetta et al. 2008; Brignani et al. 2008) (see also Table 2). Overall, the topography of the aftereffects in dependence of stimulation site is consistent with the expected generators and functional roles of the affected EPs or oscillatory components.
Direction and Size of TMS-Induced Aftereffects
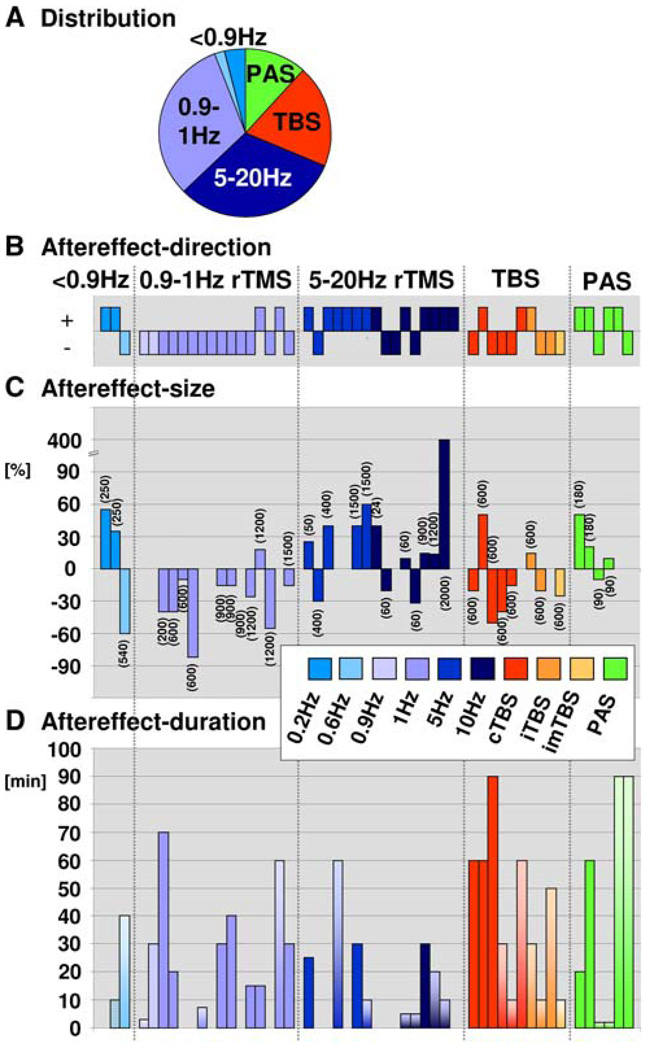
The results in regards to effect-direction (sign thereof) are illustrated in Fig. 1b for each study that observed TMS effects. Figure 1c depicts the associated effect-size (percent change relative to pre-TMS or sham) when reported or quantifiable post hoc (multiplied by effect-direction). The figures point towards a systematic difference across protocols in regards to effect-direction. Note for example in Fig. 1b, c the predominantly negative effect-direction (aftereffect of suppressive or inhibitory nature) after conventional low-frequency TMS (0.9–1 Hz) and the more frequent positive effect-directions (facilitative effects) after conventional high-frequency TMS (5–20 Hz). In contrast to effect-direction, there was a high variability in effect-size across studies (ranging from ~10–400%, absolute values, Fig. 1c), with however some tendency towards stronger effect-size with increasing number of pulses (indicated in brackets in Fig. 1c). This is further detailed next for conventional and TBS/PAS protocols separately.
Fig. 1.
Quantification of TMS-aftereffects in EEG/EPs. a Distribution of protocols. b Aftereffect-direction per study with TMS-effects. Each bar represents one experiment/study. A negative sign (−1) indexes the presence of a suppressive effect on post-TMS relative to pre-TMS or sham EEG/EPs, while a positive sign (+1) indexes facilitative effects. c Aftereffect-size (percent change in amplitude relative to pre-TMS or sham) per study reporting effect-size or from which effect-size could be calculated a posteriori (n = 37). Data are grouped according to TMS protocols (conventional TMS, TBS, PAS) and ordered according to increasing number of applied pulses (see brackets) within 5 subgroups of rTMS (0.2–0.6 Hz, 0.9–1 Hz, 5–20 Hz, TBS, PAS). d Duration of aftereffects across studies having recorded effects until recovery (n = 17, uniformly colored bars) or having reported timing information but terminated EEG recordings before its normalization (n = 21, fading color bars)
Conventional Repetitive Protocols
All but three studies reported significant aftereffects in EEG, either on amplitude (spectral power, EP-components) or latencies (EP-components, sleep stages). The three null-results were observed with low frequency stimulation (0.9, 1 Hz and 1 Hz) and can be accounted for by short stimulation duration in one case (1 Hz for 2 min, Evers et al. 2001). The other two studies with null results (Satow et al. 2003; Hansenne et al. 2004) however do not differ in design (0.9–1 Hz for 10–16.6 min) from studies with significant EEG-changes. Overall, this may suggest that the induction of aftereffects is slightly less likely with low (~1 Hz) than high TMS-frequencies (>5 Hz) when using parameters within the safety-margins. Notable is the report of aftereffects in the two studies using very low frequencies of 0.2 Hz (250 pulses, 20 min stimulation) that can be explained by the use of repeated monophasic pulses (Urushihara et al. 2006; Hosono et al. 2008), shown to be more effective than the biphasic pulse form in repetitive designs (Hosono et al. 2008).
When aftereffects were present (both amplitude and latency effects considered), there were systematic differences between low-frequency (0.9 or 1 Hz) and high-frequency protocols (5–25 Hz) in terms of aftereffect direction. Facilitation prevailed over suppression after high-frequency TMS (n = 12 vs. n = 4), and suppression prevailed over facilitation after low-frequency TMS (n = 14 vs. n = 2, Fig. 1b and Table 2). This distribution is significantly different from chance (according to a Chi-square test, P = 0.0004; all effects per experiment counted; alpha-band increase/decrease taken as sign for suppression/facilitation). Again, the studies by Urushihara et al. (2006) and Hosono et al. (2008) are noteworthy in that 0.2 Hz-stimulation with monophasic pulses evoked facilitative aftereffects, i.e. an enhancement of EP-amplitude rather than suppression.
In those studies in which percentage change from pre-TMS values or a sham control was reported or could be calculated a posteriori based on the reported data, average size of the absolute TMS-aftereffect on EEG-activity/EPs was in the order of 30% change (only amplitude—but no latency—data considered, see Table 1 and Fig. 1c). Yet, while differences in effect-directions were observed across protocols (see above), there was no such difference in terms of effect-size between low (mean = 31%, range: 10–82%; n = 11 studies) and high-frequency TMS (mean = 30%, range: 10–60%; n = 11 studies), when the only outlier (400% change; Griskova et al. 2007) was discarded.
Theta Burst (TBS) and Repeated Paired Associative Stimulation (PAS)
Only a few studies to date have explored EEG aftereffects with these protocols (TBS: Katayama and Rothwell 2007; Ishikawa et al. 2007; Sağlam et al. 2008; Schindler et al. 2008; Poreisz et al. 2008; Ortu et al. 2009; Grossheinrich et al. 2009; PAS: Tsuji and Rothwell 2002; Wolters et al. 2005; Huber et al. 2008). In these studies, both TBS and PAS were associated with significant aftereffects of effect-sizes comparable to those found following conventional rTMS (cTBS: mean = 35%, range: 15–50%, n = 6 studies; iTBS: mean = 17.5%, range: 15–20%, n = 3 studies; PAS: mean = 22.5%, range: 10–50%, n = 3 studies; see also Table 1) and provoked either facilitation or suppression (see also Fig. 1b, c).
Duration of TMS-Induced Aftereffects
Duration of EEG aftereffects on EEG-activity/EPs has been explored in a total of 38 experiments, 17 of which recorded EEG/EPs until recovery (Fig. 1d, uniformly colored bars), and with the reminder of the studies (n = 21) providing timing information but terminating the recording prior to full EEG normalization (Fig. 1d, fading color bars). The figure is suggestive of no consistent differences in aftereffect-duration across conventional protocols (Fig. 1d, blue bars), but somewhat longer aftereffect duration for TBS and PAS (Fig. 1d, red and green bars), as detailed below.
Conventional Repetitive Protocols
Absolute durations until recovery ranged from 15 to 70 min post-TMS. These aftereffect-durations did not differ between low frequency and high-frequency protocols (i.e. 0.9–1 Hz vs. 5–25 Hz rTMS: mean = 31 min vs. 28 min, range: 15–70 min vs. 25–30 min) (see Table 1 for range of employed parameters). No study so far suggests effect duration >70 min (see Fig. 1d).
Theta Burst (TBS) and Repeated Paired Associative Stimulation (PAS)
Of the 16 experiments using these protocols, 5 have estimated time of aftereffects until recovery (TBS: mean = 70 min, range: 60–90 min: n = 3; PAS: mean = 40 min, range: 20–60 min: n = 2). The duration of aftereffects seems overall higher than with conventional rTMS. Some of the studies that did not record until recovery have found aftereffects to persist at 90 min (see Fig. 1d, fading color bars), but this is based on a small number of studies and has to be investigated in more detail in the future.
Note that as compared to conventional protocols, TBS studies used similar number of pulses but considerably shorter duration and lower intensity of stimulation (Table 1) to equate risk (Katayama and Rothwell 2007; Ishikawa et al. 2007).
Prediction of Aftereffect-Size by TMS-Parameters
While there was no evidence for aftereffect-size to differ across protocols (see above), aftereffect-size correlated with several TMS-parameters.
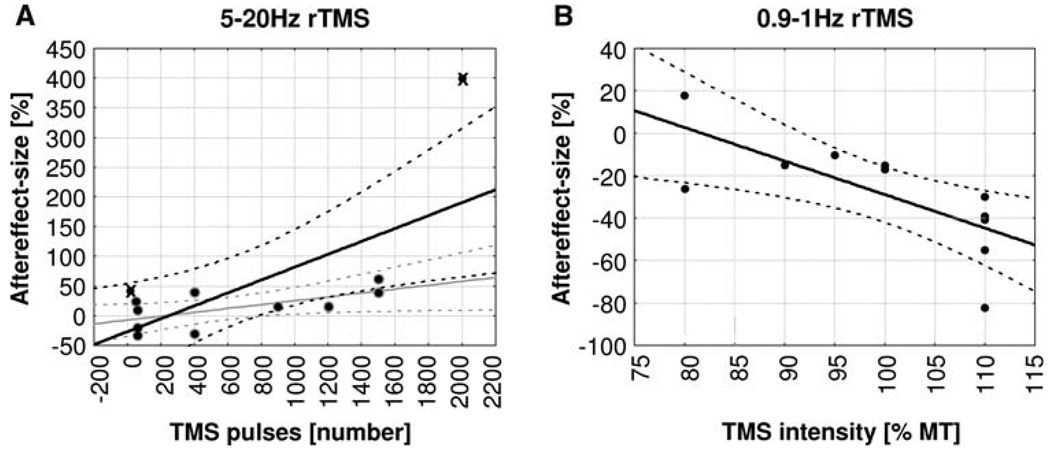
Conventional High-Frequency TMS (5–20 Hz)
Aftereffect-size depended on number of pulses applied. The effect-size significantly increased with increasing number of pulses (Pearson correlation coefficient: r = 6.7, P = 0.017, n = 12), a correlation that remained significant even when the two extremes in terms of number of pulses (n = 24 pulses: Klimesch et al. 2003, n = 2000 pulses: Griskova et al. 2007) were excluded (r = 6.3, P = 0.049, n = 10, see Fig. 2a). For high-frequency TMS, effect-size did also significantly increase with number of trains (r = 9.3, P < 0.0001 for n = 12/r = 5.87, P = 0.075 for n = 10 without Klimesch et al. 2003 and Griskova et al. 2007, results not illustrated), but did not show a significant relationship with any of the other TMS-parameters assessed (including TMS-intensity).
Fig. 2.
Linear correlations between aftereffect-size [%] and rTMS-parameters. a Aftereffect-size as a function of total number of TMS pulses applied in high-frequency protocols (5–20 Hz rTMS). Regression lines and 95% confidence intervals are shown for analyses including all data points (black lines) and excluding outliers (grey lines, outliers marked by crosses, see also text). b Aftereffect-size as a function of TMS intensity applied in low-frequency protocols (0.9–1 Hz)
Conventional Low-Frequency TMS (0.9–1 Hz)
There was a significant negative relationship between aftereffect-size and TMS-intensity (r = −0.72, P = 0.013, n = 11) indicating stronger suppressive effects with higher intensities (see Fig. 2b), but with no other TMS-parameter. Note that the dissociation of effect-size to correlate with number of pulses in high-frequency and with TMS-intensity in low-frequency applications might partially be explained by unequal variability of these two parameters across protocols. Number of pulses employed across studies was more variable for high- than low-frequency applications (standard deviation: 709 vs. 327; n = 12 vs. n = 11). Conversely, intensities employed were more variable for low- than high-frequency TMS (standard deviation: 11 vs. 8; n = 12 vs. n = 11).
No correlations between effect-size and TMS-parameters were computed for TBS and PAS due to lack of variance (see Table 1) and we did not find a correlation between effect-duration (time until recovery) and any of the TMS parameters.
Discussion
We find the electroencephalographic aftereffects of rTMS to be robust with a mean effect-size of 30–35% change from baseline or sham and a mean duration of 35 min, in addition of being absent in only a very small number of studies. Furthermore, we find the aftereffect direction (suppression vs. facilitation) to depend on the protocol employed. Conventional low-frequency (1 Hz) and high-frequency TMS (5 Hz or above) differed in terms of their suppressive versus facilitative impact on brain activity, in line with studies on rTMS-induced changes in human corticospinal motor excitability (e.g. Pascual-Leone et al. 1994; Maeda et al. 2000; Hallett 2007) as well as findings in animal models (Valero-Cabre et al. 2007). Finally, we did not find any evidence for aftereffect-size or aftereffect-duration to differ between the currently used TMS protocols (conventional rTMS, TBS, PAS). However, aftereffect-size did depend on the number of pulses, trains and intensities applied.
Aftereffect of a Single TMS-Session on EEG/EPs: Comparison with Other Measures
The observed aftereffect-size in the EEG/EPs after rolandic TMS for instance is in the range of physiological changes observed with motor learning, sustained movements, or muscle fatigue. The TMS-induced, 10–60% changes in SEP-amplitude (Enomoto et al. 2001; Tsuji and Rothwell 2002; Wolters et al. 2005; Katayama and Rothwell 2007; Ishikawa et al. 2007; Restuccia et al. 2007; Table 2) are equivalent to a 35–46% change in SEP-amplitude reported with motor skill acquisition (Nelson et al. 2001). Likewise, the TMS-induced, 10–82% changes in alpha/beta-activity (power/coherence) (Strens et al. 2002; Oliviero et al. 2003; Tamura et al. 2005; Fuggetta et al. 2008; Brignani et al. 2008; Table 2) compare in order of magnitude to changes in alpha/beta-oscillations as a consequence of sustained finger movements (10–40%: Erbil and Ungan 2007) and muscle fatigue (30–35%: Liu et al. 2005). Similarly, the TMS-induced, 30% change in the readiness-potential (RP) (Rossi et al. 2000; Table 2) is comparable to the 100% RP-change due to fatigue (Johnston et al. 2001; Schillings et al. 2006).
The observed aftereffect-duration in the EEG/EPs is furthermore comparable with the TMS-induced aftereffects on corticospinal motor excitability measured via EMG (e.g. Gerschlager et al. 2001; Münchau et al. 2002; Peinemann et al. 2004). It also matches in order of magnitude the time-course of apparent TMS-induced behavioral changes that have been assessed in studies on cognition (mostly using 1 Hz protocols), and shown to last approximately as long as the duration of the stimulation itself (Robertson et al. 2003). Based on this rule-of-thumb, we can estimate the behavioral aftereffects in the 1 Hz-TMS papers we reviewed (and for which time until recovery was assessed, n = 7) to have lasted about 15.5 min (= mean duration of stimulation, see Table 2). This compares to a measured 31 min of EEG-changes.
Overall, the outcome of currently employed repetitive TMS protocols seems thus to lie within the range of physiologic changes (learning, fatigue) and not to outlast the duration of an experiment (~1 h). Within certain limitations (see paragraph below), this contributes to further characterize the safety of repetitive TMS (Rossi et al. 2009), in particular for experiments with healthy volunteers in whom short-lived effects are desirable. However, the effect-duration in the order of a couple of minutes also limits a more versatile use of rTMS in neurotherapeutics. In the following, we will discuss the most promising ways to further prolong effect-duration when desirable, such as in clinical applications. We will also discuss very recent developments that focus on potential short-lived frequency entrainment by a single train, which is of interest for research on brain oscillations.
Estimation of rTMS-Aftereffects by EEG/EPs: Possible Limitations
As a cautionary note, we would like to mention that the estimation of effect-direction and effect-size via EEG can be confounded by several factors. Instances of enhanced EEG/EP-amplitude might not necessarily be the reflection of primarily facilitative aftereffects of the TMS-train, but could reflect a (secondary) mechanism compensating for initial inhibition. Conversely, a reduction of EEG/EP-amplitude could reflect secondary, potentially protective mechanisms against initial facilitation. Indeed, there are some reports of mixed facilitative and suppressive effects within the same experiment (but on different EEG/EP-measures) that have been explained by mechanisms of compensation (e.g. see Thut et al. 2003; Restuccia et al. 2007). Such physiological reactions of the brain to rTMS, possibly masking behavioral effects, might partially explain why EEG can detect changes when behavioral measures fail, as evidenced previously (Rossi et al. 2000; Hansenne et al. 2004; Holler et al. 2006; Ortu et al. 2009). However, it is conceivable that not only behavioral but also EEG changes might be masked by compensatory mechanisms, so that the range of changes detectable by EPs or EEG might be somewhat self-limiting, with EPs and oscillatory activity only disappearing in case of a discrete lesion. Such mechanisms of compensation might then lead to an effect-underestimation also by EEG. The likelihood of this to happen seems however smaller with EEG than with behavioral measures. In addition, the converse argument is also tenable. That is, it is conceivable that the EEG-measured aftereffects following TMS not only reflect the consequence of the direct TMS impact but also capture the rapid adaptation and compensatory responses by non-stimulated brain regions, which would lead to a multiplication rather than self-limitation of effects.
TMS-Efficacy: The Effect of Repeating rTMS Sessions
It is important to point out that there are no studies that have measured the duration of TMS-induced EEG-aftereffects following multiple repetitive TMS sessions over consecutive days. It is likely that the magnitude and duration of the EEG aftereffects will change in systematic ways over repeated sessions. Because the impact of rTMS can outlast the TMS-session (consisting of one long train in 1 Hz applications, or of consecutive short trains in high-frequency protocols, see Table 1), it is conceivable that a single TMS sessions, which may initially modify brain physiology without inducing clinically apparent signs, can be expanded into a therapeutically effective protocol through session-repetition at another day. In the same way as single-pulses or single-trains mutually interact when repeated within a session, lasting neurophysiologic effects may condition effects across TMS sessions. Indeed, Maeda et al. (2000) found that the effects of a repetitive TMS session on corticospinal excitability were greater when applied 24 h after an initial session, despite the initial effect having washed-out by then. Thus, despite the absence of any behavioral or clinical signs, there appeared to be a neurophysiologic trace of the first rTMS session that conditioned the impact of the second session as long as a day later. Such lasting effects and the resulting differential impact of consecutive sessions could have profound relevance for therapeutic applications, where daily sessions of rTMS are applied for 5 and up to 20 consecutive days (Fregni and Pascual-Leone 2007).
State-Dependency of TMS-Efficacy
A growing number of studies indicate that the effects of TMS depend on the state of neuronal activation in the targeted brain region at time of stimulation (for review see Silvanto and Pascual-Leone 2008). In human participants, single-pulse TMS has variable impact in evoking phosphenes or peripheral muscle responses depending on the pre-TMS level of electrical activity over specific recording sites (Romei et al. 2008a, b; Sauseng et al. 2008; Lepage et al. 2008) or in evoking phosphene attributes depending on the nature of pre-TMS visual adaptation (Silvanto et al. 2007a). Likewise, the effect-size or effect-direction (facilitation versus suppression of excitability) of repetitive TMS can be shaped by prior passive viewing (adaptation) protocol (Silvanto et al. 2007b), or by pre-conditioning the cortex via an rTMS-prime (Iyer et al. 2003), direct current stimulation (Siebner et al. 2004; Lang et al. 2004) or antiepileptic medication (Fregni et al. 2006), i.e. both effect-size and -direction have been shown to depend on the pre-TMS neuronal activation state. In analogy, the response of visual neurons evoked by TMS in cats can be predicted by pre-TMS neuronal activity patterns (Pasley et al. 2009). A better understanding of the neurophysiologic basis of the state-dependency of rTMS-effects is thus likely to provide new ideas how to tailor rTMS-aftereffects in desired directions.
On the one hand, the state of activity in the targeted brain region might be altered by an initial rTMS session, conditioning the outcome of a subsequent session at another day as reviewed above. On the other hand, it is conceivable that individual EEG is carrying useful information for tailoring TMS-effects to fine-tune treatment benefits in patients, given that EEG can be used to infer momentary brain state across time and participants (e.g. Thut et al. 2006; Romei et al. 2008a, b; Sauseng et al. 2008). Future TMS-EEG studies are needed to address this point. The notion of EEG-gated TMS to maximize therapeutic efficacy is appealing. Indeed, it has been shown that the impact of TMS could be maximized when TMS application would be gated by the individually measured alpha or beta rhythm (e.g. Romei et al. 2008a, b; Sauseng et al. 2008; Lepage et al. 2008). Timing the TMS to the underlying background EEG activity is likely to be critical also for the antiepileptic effects of TMS (see article by Rotenberg et al. in this issue). Therapeutic effects of TMS on depression, hallucinations, pain or stroke recovery might behave similarly.
Note on TMS-Efficacy Across Protocols
To date, only a small number of TMS-EEG studies using TBS and PAS are available. Although our review suggests there to be no major differences between conventional TMS and TBS/PAS, the latter two protocols hold more promise than conventional approaches for inducing longer lasting effects with clinical relevance. In fact, based on previous findings that behavioral changes to TBS can last up to 10 h (Nyffeler et al. 2006), longer lasting EEG-aftereffects are to be expected in future theta burst-EEG studies.
Interaction of rTMS-Frequency with Ongoing Background Activity
While the TMS-designs of the abovementioned studies with regular pulse and train repetitions do induce aftereffects >10 min on most measures of electrical brain activity (e.g. EP, sleep stages, oscillations), several recent studies have explored the potential of immediate (and likely shorter lasting) frequency entrainment at the rTMS-frequency (for a recent review, see Thut and Miniussi 2009). The question that is raised is whether a conventional, short TMS train (by virtue of its rhythmicity) will entrain background oscillations at the frequency of stimulation, if the train coincides with the neuronal frequency of the targeted brain area. This would contrast with the longer lasting (>10 min) aftereffects on brain oscillations by conventional rTMS reviewed above, which do not seem to be directly related to the stimulation frequency (and thus to be due to entrainment), because spreading to other frequency-bands (see e.g. changes in 10 Hz brain oscillations after 1 Hz-TMS; Brignani et al. 2008; Table 2). In other words, the longer lasting aftereffects seem to reflect changes in oscillatory properties of the stimulated neurons which are secondary to other, rTMS-induced neurophysiologic effects (as opposed to reflect entrainment of brain oscillations to the rhythmic TMS train).
While not much EEG information is available on the potential of rTMS to entrain rhythms, there is new evidence from behavioral studies that entrainment might indeed take place. Transcranial rhythmic stimulation has been shown to alter brain function in a frequency specific manner. For instance, performance in a task requiring visual imagery has been shown to be affected by prior parietal TMS at alpha frequency (10 Hz) (Klimesch et al. 2003). In addition, the restorative effect of sleep (Massimini et al. 2007) as well as memory consolidation (Marshall et al. 2006) have been shown to be enhanced by nocturnal transcranial rhythmic stimulation at slow wave frequency (<4 Hz). Both effects are in line with these oscillations’ suggested respective roles in visual and memory functions (e.g. Klimesch et al. 2003; Massimini et al. 2007, see also Thut and Miniussi 2009). Furthermore, because these effects of rhythmic transcranial stimulation were frequency specific, i.e. not observed with stimulation at other frequencies (Klimesch et al. 2003; Marshall et al. 2006), they are likely to be due to direct rather than secondary effects of the ryhthmic trains. This is partially supported by recent findings of a trend for behavioral effects of parietal 10 Hz/alpha rTMS to depend on individual alpha frequency (IAF) (Hamidi et al. in press).
Future studies will have to show that the frequency-specific effects on behavior are also accompanied by an enhancement of oscillatory power in the EEG at the rTMS-frequency. If so, conventional short-train rTMS is likely to develop into a powerful tool for research on the functional role and anatomical origin of brain oscillations.
Concluding Remarks
Reviewing the literature on TMS-aftereffects in EEG, we found robust effects of 1 h duration or shorter with an effect-size in the range of a physiologic change (learning or fatigue), when current protocols and single sessions are used. The observed time-range and effect-size is suitable for studies in the healthy population, but limits the therapeutic utility of these protocols. Several factors are likely to help prolong these aftereffects when desired, such as repeating sessions over days, taking into consideration pre-TMS neuronal state, and the development of new, non-conventional protocols. Recent developments also suggest that single rTMS trains might entrain neuronal rhythms at stimulation frequency, which is of interest for the study of brain oscillations. Future combined TMS-EEG work is needed to explore and extend the utility of rTMS in both research and therapy.
Contributor Information
Gregor Thut, Centre for Cognitive Neuroimaging, Department of Psychology, University of Glasgow, 58 Hillhead Street, Glasgow G12-8QB, UK, g.thut@psy.gla.ac.uk.
Alvaro Pascual-Leone, Berenson-Allen Center for Noninvasive Brain Stimulation, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Avenue, Boston, MA 02215, USA.
References
- Bae EH, Schrader LM, Machii K, Alonso-Alonso M, Riviello JJ, Jr, Pascual-Leone A, Rotenberg A. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav. 2007;10:521–528. doi: 10.1016/j.yebeh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Bohotin V, Fumal A, Vandenheede M, Gérard P, Bohotin C, Maertens de Noordhout A, Schoenen J. Effects of repetitive transcranial magnetic stimulation on visual evoked potentials in migraine. Brain. 2002;125:912–922. doi: 10.1093/brain/awf081. [DOI] [PubMed] [Google Scholar]
- Brignani D, Manganotti P, Rossini PM, Miniussi C. Modulation of cortical oscillatory activity during transcranial magnetic stimulation. Hum Brain Mapp. 2008;29:603–612. doi: 10.1002/hbm.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Classen J, Wassermann EM, Hallett M, Cohen LG. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol. 1997;105:415–421. doi: 10.1016/s0924-980x(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Chen WH, Mima T, Siebner HR, Oga T, Hara H, Satow T, Begum T, Nagamine T, Shibasaki H. Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin Neurophysiol. 2003;114:1628–1637. doi: 10.1016/s1388-2457(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Classen J, Wolters A, Stefan K, Wycislo M, Sandbrink F, Schmidt A, Kunesch E. Paired associative stimulation. Suppl Clin Neurophysiol. 2004;57:563–569. [PubMed] [Google Scholar]
- Cohrs S, Tergau F, Riech S, Kastner S, Paulus W, Ziemann U, Rüther E, Hajak G. High-frequency repetitive transcranial magnetic stimulation delays rapid eye movement sleep. Neuroreport. 1998;9:3439–3443. doi: 10.1097/00001756-199810260-00019. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Ugawa Y, Hanajima R, Yuasa K, Mochizuki H, Terao Y, Shiio Y, Furubayashi T, Iwata NK, Kanazawa I. Decreased sensory cortical excitability after 1 Hz rTMS over the ipsilateral primary motor cortex. Clin Neurophysiol. 2001;112:2154–2158. doi: 10.1016/s1388-2457(01)00667-8. [DOI] [PubMed] [Google Scholar]
- Erbil N, Ungan P. Changes in the alpha and beta amplitudes of the central EEG during the onset, continuation, and offset of long-duration repetitive hand movements. Brain Res. 2007;1169:44–56. doi: 10.1016/j.brainres.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69:86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Evers S, Böckermann I, Nyhuis PW. The impact of transcranial magnetic stimulation on cognitive processing: an event-related potential study. Neuroreport. 2001;12:2915–2918. doi: 10.1097/00001756-200109170-00032. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Otachi P, Thut G, Rigonatti SP, Marcolin MA, Fecteau S, Pascual-Leone A, Fiore L, Valente K. Homeostatic effects of plasma valproate levels on corticospinal excitability changes induced by 1 Hz rTMS in patients with juvenile myoclonic epilepsy. Clin Neurophysiol. 2006;117:1217–1227. doi: 10.1016/j.clinph.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Pavone EF, Fiaschi A, Manganotti P. Acute modulation of cortical oscillatory activities during short trains of high-frequency repetitive transcranial magnetic stimulation of the human motor cortex: a combined EEG and TMS study. Hum Brain Mapp. 2008;29:1–13. doi: 10.1002/hbm.20371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumal A, Bohotin V, Vandenheede M, Seidel L, de Pasqua V, de Noordhout AM, Schoenen J. Effects of repetitive transcranial magnetic stimulation on visual evoked potentials: new insights in healthy subjects. Exp Brain Res. 2003;150:332–340. doi: 10.1007/s00221-003-1423-7. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Graf T, Engeler J, Achermann P, Mosimann UP, Noss R, Fisch HU, Schlaepfer TE. High frequency repetitive transcranial magnetic stimulation (rTMS) of the left dorsolateral cortex: EEG topography during waking and subsequent sleep. Psychiatry Res. 2001;107:1–9. doi: 10.1016/s0925-4927(01)00083-x. [DOI] [PubMed] [Google Scholar]
- Griskova I, Ruksenas O, Dapsys K, Herpertz S, Höppner J. The effects of 10 Hz repetitive transcranial magnetic stimulation on resting EEG power spectrum in healthy subjects. Neurosci Lett. 2007;419:162–167. doi: 10.1016/j.neulet.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Grossheinrich N, Rau A, Pogarell O, Hennig-Fast K, Reinl M, Karch S, Dieler A, Leicht G, Mulert C, Sterr A, Padberg F. Theta burst stimulation of the prefrontal cortex: safety and impact on cognition, mood, and resting electroencephalogram. Biol Psychiatry. 2009;65:778–784. doi: 10.1016/j.biopsych.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Slagter HA, Tononi G, Postle BR. Repetitive transcranial magnetic stimulation affects behavior by biasing endogenous cortical oscillations. Front Integr Neurosci. doi: 10.3389/neuro.07.014.2009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansenne M, Laloyaux O, Mardaga S, Ansseau M. Impact of low frequency transcranial magnetic stimulation on event-related brain potentials. Biol Psychol. 2004;67:331–341. doi: 10.1016/j.biopsycho.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Holler I, Siebner HR, Cunnington R, Gerschlager W. 5 Hz repetitive TMS increases anticipatory motor activity in the human cortex. Neurosci Lett. 2006;392:221–225. doi: 10.1016/j.neulet.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Hosono Y, Urushihara R, Harada M, Morita N, Murase N, Kunikane Y, Shimazu H, Asanuma K, Uguisu H, Kaji R. Comparison of monophasic versus biphasic stimulation in rTMS over premotor cortex: SEP and SPECT studies. Clin Neurophysiol. 2008;119:2538–2545. doi: 10.1016/j.clinph.2008.07.279. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Määttä S, Esser SK, Sarasso S, Ferrarelli F, Watson A, Ferreri F, Peterson MJ, Tononi G. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J Neurosci. 2008;28:7911–7918. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Matsunaga K, Nakanishi R, Kawahira K, Murayama N, Tsuji S, Huang YZ, Rothwell JC. Effect of theta burst stimulation over the human sensorimotor cortex on motor and somatosensory evoked potentials. Clin Neurophysiol. 2007;118:1033–1043. doi: 10.1016/j.clinph.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:10867–10872. doi: 10.1523/JNEUROSCI.23-34-10867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Takigawa M. Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:1620–1631. doi: 10.1016/s1388-2457(00)00357-6. [DOI] [PubMed] [Google Scholar]
- Jing H, Takigawa M, Hamada K, Okamura H, Kawaika Y, Yonezawa T, Fukuzako H. Effects of high frequency repetitive transcranial magnetic stimulation on P(300) event-related potentials. Clin Neurophysiol. 2001a;112:304–313. doi: 10.1016/s1388-2457(00)00540-x. [DOI] [PubMed] [Google Scholar]
- Jing H, Takigawa M, Okamura H, Doi W, Fukuzako H. Comparisons of event-related potentials after repetitive transcranial magnetic stimulation. J Neurol. 2001b;248:184–192. doi: 10.1007/s004150170224. [DOI] [PubMed] [Google Scholar]
- Johnston J, Rearick M, Slobounov S. Movement-related cortical potentials associated with progressive muscle fatigue in a grasping task. Clin Neurophysiol. 2001;112:68–77. doi: 10.1016/s1388-2457(00)00452-1. [DOI] [PubMed] [Google Scholar]
- Katayama T, Rothwell JC. Modulation of somatosensory evoked potentials using transcranial magnetic intermittent theta burst stimulation. Clin Neurophysiol. 2007;118:2506–2511. doi: 10.1016/j.clinph.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur J Neurosci. 2003;17:1129–1133. doi: 10.1046/j.1460-9568.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Komssi S, Kähkönen S. The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain Res Rev. 2006;52:183–192. doi: 10.1016/j.brainresrev.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004;56:634–639. doi: 10.1016/j.biopsych.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lepage JF, Saint-Amour D, Théoret H. EEG and neuronavigated single-pulse TMS in the study of the observation/execution matching system: are both techniques measuring the same process? J Neurosci Methods. 2008;175:17–24. doi: 10.1016/j.jneumeth.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Yao B, Siemionow V, Sahgal V, Wang X, Sun J, Yue GH. Fatigue induces greater brain signal reduction during sustained than preparation phase of maximal voluntary contraction. Brain Res. 2005;1057:113–126. doi: 10.1016/j.brainres.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006;117:455–471. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadoóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ, Tononi G. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci. 2007;104:8496–8501. doi: 10.1073/pnas.0702495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniussi C, Thut G. Combining TMS and EEG Offers New Prospects in Cognitive Neuroscience. Brain Topogr. 2009 doi: 10.1007/s10548-009-0083-8. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Münchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci. 2002;22:554–561. doi: 10.1523/JNEUROSCI.22-02-00554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJ, Brooke JD, McIlroy WE, Bishop DC, Norrie RG. The gain of initial somatosensory evoked potentials alters with practice of an accurate motor task. Brain Res. 2001;890:272–279. doi: 10.1016/s0006-8993(00)03136-x. [DOI] [PubMed] [Google Scholar]
- Nyffeler T, Wurtz P, Lüscher HR, Hess CW, Senn W, Pflugshaupt T, von Wartburg R, Lüthi M, Mü ri RM. Extending lifetime of plastic changes in the human brain. Eur J Neurosci. 2006;24:2961–2966. doi: 10.1111/j.1460-9568.2006.05154.x. [DOI] [PubMed] [Google Scholar]
- Okamura H, Jing H, Takigawa M. EEG modification induced by repetitive transcranial magnetic stimulation. J Clin Neurophysiol. 2001;18:318–325. doi: 10.1097/00004691-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Strens LH, Di Lazzaro V, Tonali PA, Brown P. Persistent effects of high frequency repetitive TMS on the coupling between motor areas in the human. Exp Brain Res. 2003;149:107–113. doi: 10.1007/s00221-002-1344-x. [DOI] [PubMed] [Google Scholar]
- Ortu E, Ruge D, Deriu F, Rothwell JC. Theta Burst Stimulation over the human primary motor cortex modulates neural processes involved in movement preparation. Clin Neurophysiol. 2009;120:1195–1203. doi: 10.1016/j.clinph.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Allen EA, Freeman RD. State-dependent variability of neuronal responses to transcranial magnetic stimulation of the visual cortex. Neuron. 2009;62:291–303. doi: 10.1016/j.neuron.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor MA, Thut G, Pascual-Leone A. Modulation of steady-state auditory evoked potentials by cerebellar rTMS. Exp Brain Res. 2006;175:702–709. doi: 10.1007/s00221-006-0588-2. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Löer C, Quartarone A, Münchau A, Conrad B, Siebner HR. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol. 2004;115:1519–1526. doi: 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Poreisz C, Antal A, Boros K, Brepohl N, Csifcsaók G, Paulus W. Attenuation of N2 amplitude of laser-evoked potentials by theta burst stimulation of primary somatosensory cortex. Exp Brain Res. 2008;185:611–621. doi: 10.1007/s00221-007-1188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Becker M, Tegenthoff M, Pleger B, Dinse HR. Sustained increase of somatosensory cortex excitability by 5 Hz repetitive transcranial magnetic stimulation studied by paired median nerve stimulation in humans. Neurosci Lett. 2004;356:91–99. doi: 10.1016/j.neulet.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Restuccia D, Ulivelli M, De Capua A, Bartalini S, Rossi S. Modulation of high-frequency (600 Hz) somatosensory-evoked potentials after rTMS of the primary sensory cortex. Eur J Neurosci. 2007;26:2349–2358. doi: 10.1111/j.1460-9568.2007.05828.x. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Théoret H, Pascual-Leone A. Studies in cognition: the problems solved and created by transcranial magnetic stimulation. J Cogn Neurosci. 2003;15:948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- Rollnik JD, Schröder C, Rodríguez-Fornells A, Kurzbuch AR, Däuper J, Möller J, Münte TF. Functional lesions and human action monitoring: combining repetitive transcranial magnetic stimulation and event-related brain potentials. Clin Neurophysiol. 2004;115:145–153. doi: 10.1016/j.clinph.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008a;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Rihs T, Brodbeck V, Thut G. Resting electroencephalogram alpha-power over posterior sites indexes baseline visual cortex excitability. Neuroreport. 2008b;19:203–208. doi: 10.1097/WNR.0b013e3282f454c4. [DOI] [PubMed] [Google Scholar]
- Rossi S, Rossini PM. TMS in cognitive plasticity and the potential for rehabilitation. Trends Cogn Sci. 2004;8:273–279. doi: 10.1016/j.tics.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Rossini PM, Feige B, Ulivelli M, Glocker FX, Battistini N, Lucking CH, Kristeva-Feige R. Effects of repetitive transcranial magnetic stimulation on movement-related cortical activity in humans. Cereb Cortex. 2000;10:802–808. doi: 10.1093/cercor/10.8.802. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009 doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sağlam M, Matsunaga K, Murayama N, Hayashida Y, Huang YZ, Nakanishi R. Parallel inhibition of cortico-muscular synchronization and cortico-spinal excitability by theta burst TMS in humans. Clin Neurophysiol. 2008;119:2829–2838. doi: 10.1016/j.clinph.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Satow T, Mima T, Yamamoto J, Oga T, Begum T, Aso T, Hashimoto N, Rothwell JC, Shibasaki H. Short-lasting impairment of tactile perception by 0.9 Hz-rTMS of the sensorimotor cortex. Neurology. 2003;60:1045–1047. doi: 10.1212/01.wnl.0000052821.99580.d3. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gerloff C, Hummel FC. Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2008.07.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Schillings ML, Kalkman JS, Van Der Werf SP, Bleijenberg G, van Engelen BG, Zwarts MJ. Central adaptations during repetitive contractions assessed by the readiness potential. Eur J Appl Physiol. 2006;97:521–526. doi: 10.1007/s00421-006-0211-z. [DOI] [PubMed] [Google Scholar]
- Schindler K, Nyffeler T, Wiest R, Hauf M, Mathis J, Hess ChW, Müri R. Theta burst transcranial magnetic stimulation is associated with increased EEG synchronization in the stimulated relative to unstimulated cerebral hemisphere. Neurosci Lett. 2008;436:31–34. doi: 10.1016/j.neulet.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J. Reductions in CI amplitude after repetitive transcranial magnetic stimulation (rTMS) over the striate cortex. Brain Res Cogn Brain Res. 2003;16:488–491. doi: 10.1016/s0926-6410(02)00300-2. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J, d’Alfonso AA, Postma A, de Haan EH. Effects of slow rTMS at the right dorsolateral prefrontal cortex on EEG asymmetry and mood. Neuroreport. 2001;12:445–447. doi: 10.1097/00001756-200103050-00005. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J, d’Alfonso AA, Peper JS, Panksepp J. High frequency repetitive transcranial magnetic over the medial cerebellum induces a shift in the prefrontal electroencephalography gamma spectrum: a pilot study in humans. Neurosci Lett. 2003;336:73–76. doi: 10.1016/s0304-3940(02)01077-7. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural adaptation reveals state-dependent effects of transcranial magnetic stimulation. Eur J Neurosci. 2007a;25:1874–1881. doi: 10.1111/j.1460-9568.2007.05440.x. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural activation state determines behavioral susceptibility to modified theta burst transcranial magnetic stimulation. Eur J Neurosci. 2007b;26:523–528. doi: 10.1111/j.1460-9568.2007.05682.x. [DOI] [PubMed] [Google Scholar]
- Strens LH, Oliviero A, Bloem BR, Gerschlager W, Rothwell JC, Brown P. The effects of subthreshold 1 Hz repetitive TMS on cortico-cortical and interhemispheric coherence. Clin Neurophysiol. 2002;113:1279–1285. doi: 10.1016/s1388-2457(02)00151-7. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Hoshiyama M, Nakata H, Hiroe N, Inui K, Kaneoke Y, Inoue K, Kakigi R. Functional relationship between human rolandic oscillations and motor cortical excitability: an MEG study. Eur J Neurosci. 2005;21:2555–2562. doi: 10.1111/j.1460-9568.2005.04096.x. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Walsh V, Eimer M. Combining TMS and EEG to study cognitive function and cortico–cortico interactions. Behav Brain Res. 2008;191:141–147. doi: 10.1016/j.bbr.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Miniussi C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn Sci. 2009;13:182–189. doi: 10.1016/j.tics.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Thut G, Théoret H, Pfennig A, Ives J, Kampmann F, Northoff G, Pascual-Leone A. Differential effects of low-frequency rTMS at the occipital pole on visual-induced alpha desynchronization and visual-evoked potentials. Neuroimage. 2003;18:334–347. doi: 10.1016/s1053-8119(02)00048-4. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Rothwell JC. Long lasting effects of rTMS and associated peripheral sensory input on MEPs, SEPs and transcortical reflex excitability in humans. J Physiol. 2002;540:367–376. doi: 10.1113/jphysiol.2001.013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara R, Murase N, Rothwell JC, Harada M, Hosono Y, Asanuma K, Shimazu H, Nakamura K, Chikahisa S, Kitaoka K, Sei H, Morita Y, Kaji R. Effect of repetitive transcranial magnetic stimulation applied over the premotor cortex on somatosensory-evoked potentials and regional cerebral blood flow. Neuroimage. 2006;31:699–709. doi: 10.1016/j.neuroimage.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, Pascual-Leone A. Opposite impact on (14) C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res. 2007;176:603–615. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Paus T. The neural response to transcranial magnetic stimulation of the human motor cortex. I. Intracortical and cortico-cortical contributions. Exp Brain Res. 2006;175:231–245. doi: 10.1007/s00221-006-0551-2. [DOI] [PubMed] [Google Scholar]
- Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nat Rev Neurosci. 2000;1:73–79. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, Benecke R, Reiners K, Classen J. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–1052. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]