Figure 2.
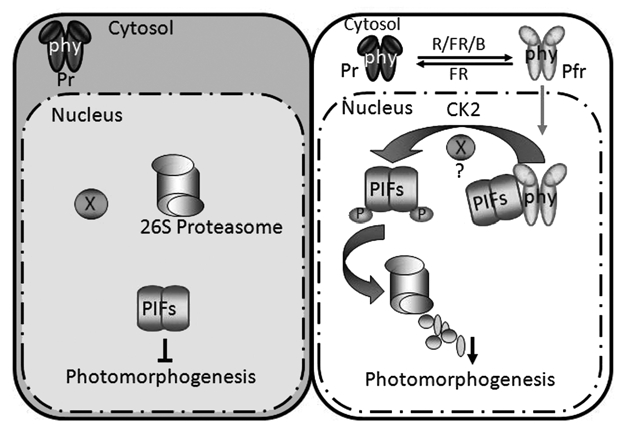
Simplified model demonstrating the antagonistic relationship between phys and PIFs. (Left) In the dark, phys are present in the cytosol as an inactive Pr form, while PIFs are constitutively localized into the nucleus repressing photomorphogenesis. (Right) phys perceive the light signal that induces photo-conversion to the active Pfr form and nuclear migration. Within the nucleus, the Pfr form of phys physically associates with PIFs and induces rapid phosphorylation of PIFs in response to light. CK2 also phosphorylates PIF1 and possibly other PIFs independent of light. The dual phosphorylation (separately by CK2- and an unknown light-inducible kinase-mediated) of PIF1 and possibly other PIFs promotes poly-ubiquitylation and 26S proteasome-mediated degradation in response to light. The light-induced degradation of PIFs relieves the repressive action to promote photomorphogenesis. X, indicates an unknown kinase that phosphorylates PIFs in response to light. P, phosphorylated form. This figure has been adapted and modified from Castillon et al.6
