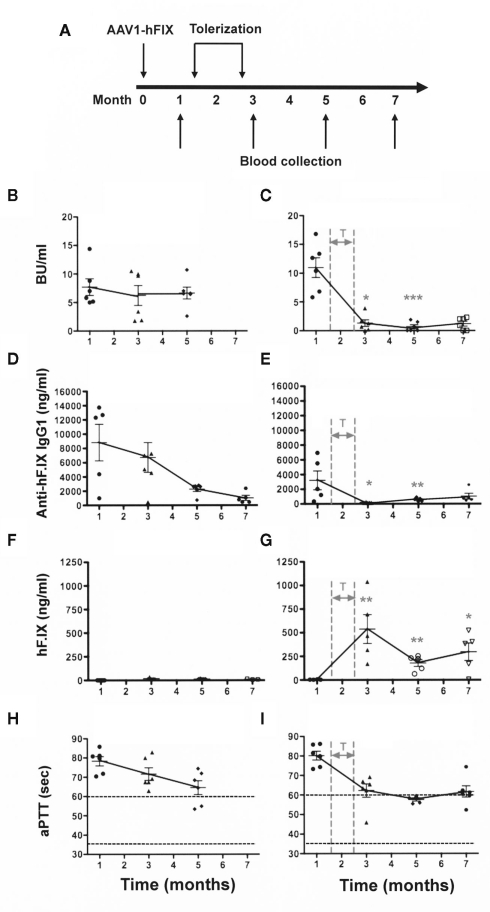
Figure 1.
Elimination of a pre-existing/ongoing anti-hF.IX immune response. C3H/HeJ F9−/− mice (n = 12) were injected IM with AAV1-CMV-hF.IX (1 × 1011 vg/mouse) to generate an immune response. After demonstrating the existence of an immune response, half of the animals were given rapamycin/IL-10/specific peptide for 4 weeks [right panels: (C,E,G,I)]. Controls [left panels: (B,D,F,H)] only received gene transfer. (A) Experimental timeline. (B,C) Inhibitory antibody titers (in BU/ml). (D,E) Anti-hF.IX IgG1 antibody (ng/ml). (H,G) hF.IX plasma levels (ng/ml). (H,I) Coagulation times (aPTT in s). Data are shown as a function of time after vector administration in control mice (treated with vector only, left panels) or mice that received the immune modulation/tolerance regimen (starting 1.5 months after vector administration, indicated as “T” in right panels). Results are shown as averages at each time point (±SEM) and for individual animals. Statistically significant differences between the immune modulated and control groups (n = 6 per group) at a specific time point are indicated as gray asterisk in the panels of the immune modulated group with *P < 0.05, **P < 0.01, and ***P < 0.001.