Figure 2.
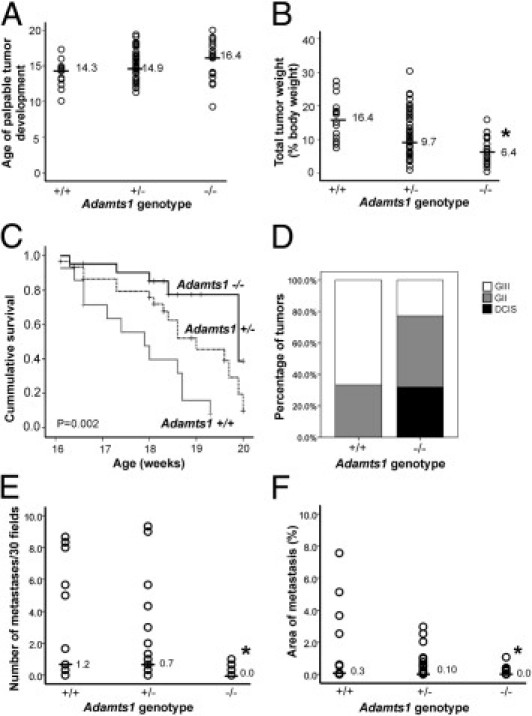
Tumor progression is reduced in Adamts1−/−/PyMT mice. ADAMTS1-deficient mice develop tumors at similar age to controls, but showed reduced primary mammary tumor burden, increased survival to ethically mandated euthanasia and lower histopathological grade and pulmonary metastasis. A: Age of first palpable tumor observation. P = 0.071, Kruskal-Wallis test. B: Total tumor weight normalized to body weight in Adamts1+/+, Adamts1+/−, and Adamts1−/−/PyMT+ cohorts. *P < 0.0001, Kruskal-Wallis test. Horizontal lines indicate the median for each cohort (A and B). C: Survival to ethically mandated euthanasia in Adamts1/PyMT+ mice cohorts (Mantel-Cox log-rank test statistic 13.58, P = 0.001). D: Proportion of DCIS, grade II (GII), and grade III (GIII) invasive tumors in Adamts1+/+ (n = 15) and Adamts1−/−/PyMT+ mice (n = 21). E: Number of lung metastases identified in 30 random fields from serial sections spanning lung tissue at 100-μm increments in Adamts1/PyMT mouse cohorts. *P = 0.016, Kruskal-Wallis test. F: Percentage of lung area containing metastases in serial sections spanning the tissue at 100-μm increments of Adamts1/PyMT mice cohorts. *P = 0.028, Kruskal-Wallis test.
