Figure 3.
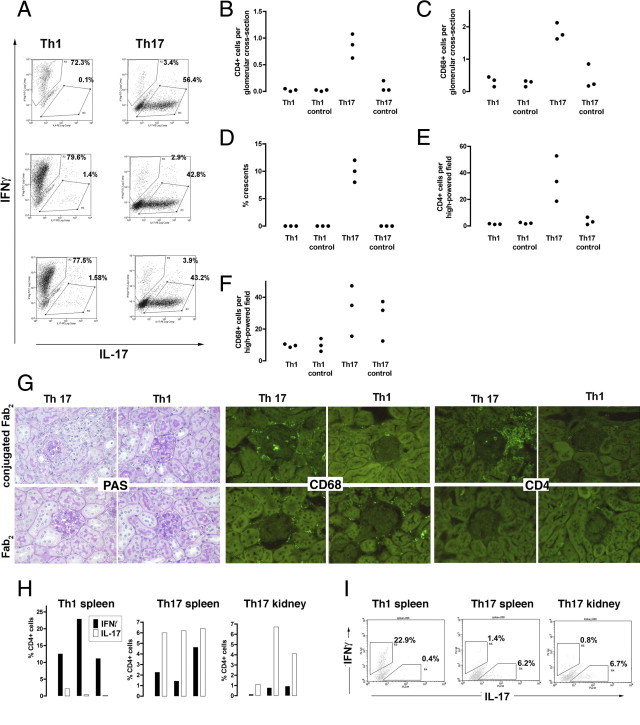
A: OT2 × RAG1−/− splenocytes were cultured in polarizing conditions and FACS staining showed that CD4+ cells of the desired polarity were obtained using these methods. The plots shown are from three independently cultured cell lines (derived from different mice) of each type that were used in this experiment. B–F: Polarized TH1 or TH17 CD4+ T cells were given to RAG1−/− mice followed by an injection of peptide-conjugated Fab2 nephrotoxic antibody or unconjugated Fab2 antibody. Each mouse in each group was given a different cell line and so is an independent data point, and each symbol is an individual mouse. The data show that at day 21 glomerular CD68+ macrophage and CD4+ T-cell infiltration with crescent formation developed only in mice given peptide-conjugated antibody and TH17 cells but not in mice given TH17 or TH1 cells with unconjugated Fab2 or in mice given TH1 cell with peptide-conjugated Fab2 (P < 0.001 for all comparisons of glomerular histologic analysis). The interstitial CD4+ cell infiltrate was also increased in this group (P < 0.01). The interstitial macrophage infiltrate was greater after TH17 cells in both the presence and absence of peptide (but not significant when all four groups were compared). G: Representative histologic analysis on PAS-stained sections from the experiment shown in B–F, with immunofluorescence staining for CD68+ macrophages and CD4+ cells. The glomeruli are darker than the surrounding autofluorescent tubules. H: Intracellular cytokine staining to examine the polarity of CD4+ T cells in mice that had been given TH1 or TH17 cells and peptide-conjugated Fab2. Each bar is a separate mouse from the experiment depicted. We also digested kidneys from mice that had been given TH17 cells and peptide-conjugated Fab2 and performed intracellular cytokine staining on isolated CD4+ T cells, which showed that they had retained their polarity. No data are shown for the other groups because kidneys did not contain significant T-cell numbers (B and E). I: Representative flow cytometry plots gated on CD4+ cells. Although the percentages are lower than those seen in the original cell lines (A), this probably represents technical issues around isolating splenic and renal T cells and performing intracellular cytokine staining, and the data clearly show that polarity had been retained.
