Figure 7.
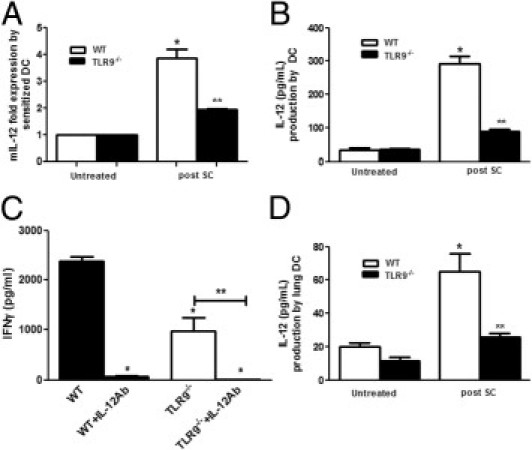
Production of IL-12 by sensitized bone marrow DCs and lung DCs harvested from WT and TLR9−/− and antigen presentation by WT and TLR9−/− DCs. WT and TLR9−/− mice were sensitized by SC, and bone marrow cells were harvested at day 0, cells were cultured with GM-CSF, and DCs were purified by CD11c+ magnetic beads at day 6 and stimulated with SC spores (1:10). IL-12 protein levels were measured by ELISA in supernatant fluids collected at 18 hours after stimulation (B) and IL-12 p40 mRNA expression was measured by real-time PCR (A). In a separate experiment, sensitized bone marrow DCs from WT and TLR9−/− mice were cocultured with naive WT splenic T cells for 18 hours, and their antigen-presenting function was measured by T-cell IFN-γ production in the presence or absence of IL-12 antibody by ELISA (C). Lung DCs were harvested from sensitized WT and TLR9−/− mice as described previously and stimulated with SC, and cell supernatant levels of IL-12 were measured by ELISA (D). N = 4 to 5 in each group; each experiment was repeated twice. Results represent mean ± SEM. *P < 0.05 compared with untreated controls; **P < 0.05 compared with WT mice after SC or *P <0.01 compared with IL-12 antibody (Ab) treatment.
