Abstract
Background:
Fructus Corni is derived from the dry ripe sarcocarp of Cornus officinalis Sieb. et Zucc. 5-hydroxymethyl-2-furaldehyde (5-HMF) is an important active composition of the Fructus Corni. However, there have been no reports on the concentration of 5-HMF in freely moving rats using microdialysis coupled with HPLC.
Materials and Methods:
The concentration of 5-HMF in free-moving rats after intra-gastric (i.g.) administration of the water extract of Fructus Corni and JZP was analyzed by microdialysis coupled with high-performance liquid chromatographic (HPLC).
Results:
Results demonstrated that the concentration of 5-HMF in microdialysate was 1.4951 μg/l, but higher in rat microdialysate after i.g. administration of the aqueous extract of JZP (5.2662 μg/l).
Conclusion:
This method is proved to be rapid, accurate and simple. Real-time in vivo monitoring the concentration of 5-HMF provides the theoretical basis for further explaining the processing mechanism of Fructus Corni.
Keywords: 5-HMF, fructus corni, high performance liquid Chromatography with diode array detector, microdialysis
INTRODUCTION
Traditional Chinese medicines (TCMs) have been used by TCMs practitioners for thousands of years. They have been attracting ever-increasing attention for their therapeutic effects to Western medicines with few side effects.[1,2] Although many TCMs have been proven effective by modern pharmacological studies and clinical trials, their bioactive components and the remedial mechanism are still not well understood. So far it is widely accepted that TCMs are mostly used in combination and the composite formulae will produce a synergistic effect or antagonistic action. So, the clarification of bioactive ingredients of TCMs needs an integrative method that can make it possible to perform bioactive assay, chemical isolation, and identification of captured compounds almost simultaneously.[3]
Fructus Corni is derived from the dry ripe sarcocarp of Cornus officinalis Sieb. et Zucc. The crude drug and its JZP are used clinically for nourishing liver and kidney.[4] It has been increasingly paid much attention as one of the most popular and cherished herbal medicine in clinic in the world and can be used for medicine, hygienic food and cosmetic due to its biological and phamacyological actives, such as anti-inflammation, anti-virous, and anti-oxidation and so on.[5,6] Five-HMF is an endogenous product found in plants, and in free or bound forms, it is found in large amounts in processed Fructus Corni from which it is extracted in hot aqueous infusions. Pharmacological studies on the components showed that 5-HMF had good biological activities, such as anti-inflammatory activity and bacteriostatic action.[7–9] Recent studies found that 5-HMF of the Fructus Corni has great protection effect to the CC14 liver injury and the vascular endothelial cell, therefore, it is believed that 5-HMF is an important active composition of the Fructus Corni.[10] The researcher also found that 5-HMF in the JZP is much more than 5-HMF in the crude drugs, this improves that steaming can increase the 5-HMF. Besides the researchers found 5-HMF from the Fructus Corni which is boiled in aqueous is three times higher than that from the methanol ultrasonic extraction, but there is little difference in the preparation. It is concluded that heating has great effect to the amount of the 5-HMF, however, the researchers has not made the study about how the 5-HMF in the aqueous extract of crude Fructus Corni and JZP changes in body.
Intra-gastric (i.g.) administration is often considered as a safe and acceptable route of drug delivery of blood, gaining significant recent interest, especially for the substances with biological effects on the liver and kidney.[11,12] Considerable studies to present have been undertaken on liver and kidney delivery system. Therefore, a design using a freely moving animal model in the study is also suggested to be employed due to an effect of the anesthetic agents to a variable degree on the Intra-gastric absorption, in order to make the experimental data on blood delivery more conformable to physiological condition.
Recently, microdialysis coupled with HPLC has developed as one of the most powerful analytical techniques. Microdialysis is a continuous sampling technique [Figure 1], therefore, each sample represents the average concentration of analyte in the blood during the sampling interval. This is compared to taking discrete blood samples which represent the concentration in the blood only at the time of sampling.[13,14] Because of the continuous sampling, microdialysis is an integrating technique which is less prone to fluctuations than an instantaneous sampling technique. In present paper, microdialysis coupled with HPLC-DAD was utilized to study the concentrations of 5-HMF in freely moving rats after i.g. administration of the aqueous extract of crude drug and JZP. To our knowledge, there have been no reports on the concentration of 5-HMF in freely moving rats using microdialysis coupled with HPLC. A novel method was developed to study the process mechanism of TCM.
Figure 1.
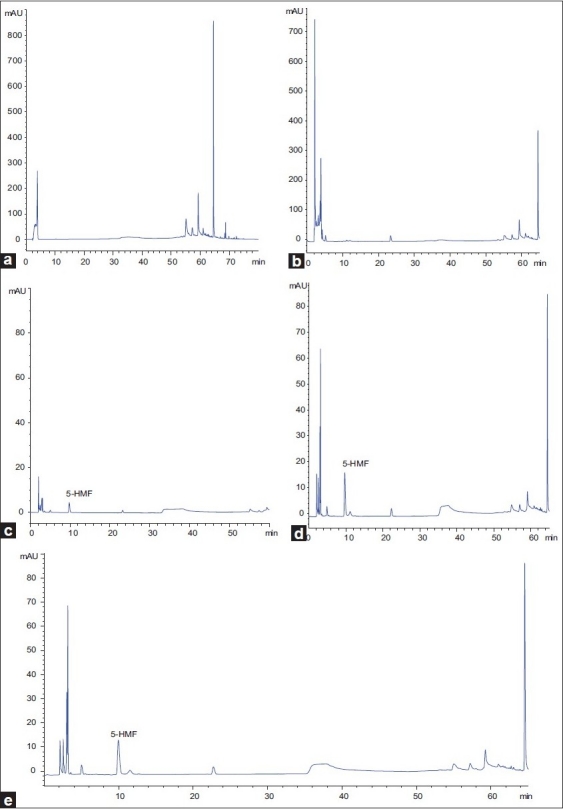
HPLC Chromatograms of 5-HMF in freely moving rat samples a) blank ACD solutionusp; b) blank dialysate sample; c) dialysate sample after i.g. administration of the aqueous extract of crud Fructus corni; d) dialysate sample after i.g. administration of the aqueous extract of JZP; e) blank dialysate sample with standard 5-HMF
MATERIALS AND METHODS
Materials
The processed Fructus Corni of jiu zheng pin (JZP) was collected from Henan suppliers. Five-HMF was prepared from our laboratory and its identity was verified by LC-MS, 1HNMR, and 13CNMR.[15] The purity for each standard compound was greater than 98% by HPLC analysis. Acetonitrile was purchased from E.Merck (Darmstadt, Germany). Deionized aqueous was purified by Milli-Q system (Millipore, Bedford, MA, USA). Solvents (Millipore Corp., Bedford, MA) were of HPLC grade used for all preparations. All reagents with high grade were obtained from others.
Animals
Twelve male adult Sprague-Dawley rats weighing approximately 300 g were obtained from the Laboratory Animal Center of Zhejiang Chinese Medical University (Zhejiang, China). Animals were acclimatized for at least five days with alternating dark/light cycle of each 12 h in a climate controlled room with temperature maintained at 22 ± 1°C and a relative humidity of 60 ± 10%. Water and standard laboratory food were available ad libitum. All experiments were performed according to the guidelines for the care and use of animals as established by Zhejiang Chinese Medical University.
Preparation of sample solutions
The powder of Fructus Corni and JZP samples quantitatively (20 g) transferred into round-bottomed flask and extracted with 10 times of distilled water for about 2 h and cooled at room temperature; the solid residue was removed by filtration. The residue was boiled again with 10 times of distilled water for 1 h and, after cooling, was followed by filtration. The final volume was 18 ml.
Atomic absorption spectroscopic (AAS) analysis of five toxic heavy metals, including arsenic, cadmium, chromium, lead and mercury was performed on Thermo SOlAARS2 atomic absorption spectrometer with autosampler.
Preparation of anticoagulant citrate dextrose solution
Anticoagulant citrate dextrose solution (ACD) consisting of 0.67 g Na3C6H5O7.2H2O, 0.24 g C6H8O7.H2O, 1.50 g C6H12O6, adjusted to pH 7.4 was used as perfusion medium for microdialysis probes.
Preparation of standard solutions
A standard stock solution of 5-HMF was prepared by dissolving 6.03 mg of 5-HMF in 10 ml of ACD solution for use in sample analysis. The standard stock solution was further diluted with ACD solution to make seven different concentrations including 1, 1/4, 1/5, 1/4, 3/20, 1/10, 1/20, and 1/40 of the original concentration.
High-performance liquid chromatographic analysis
Concentrations of 5-HMF was determined by using Agilent 1200 HPLC system with diode array detector and a chemsatation data analysis system. An Agilent Zorbax Extend C18 column (250 × 4.6 mm i.d., 5 μm) was utilized as the analytical column and column temperature was maintained at 30°C. The mobile phase consisted of 2% acetonitrile and 98% of aqueous phosphoric acid (0.1%, v/v). The detector wave length was set at 284 nm. All samples were centrifugated at 15000 × g for 10 min. An injection volume of 20 μl was used.
Surgical procedures
Sprague-Dawley rats were anesthetized with ketamine/xylazine (90 mg/kg ± 10 mg/kg) by intraperitoneal injection and mounted on a stereotaxic frame (Bioanalytical Systems, West Lafayette, IN, USA). A flexible vascular microdialysis probe (MD-2310, BAS, West Lafayette, IN, USA) with characteristics of 0.5 mm O.D., 10 mm membrane length and nominal 18 kDa MW cut-off was inserted into the right jugular vein of pentobarbital-anesthetized rat towards the right atrium and perfused with perfusion fluid. A flexible wire mesh on the probe is sutured to the pectoral muscle. Inlet and outlet lines to the probe are housed within a single piece of flexible tubing, which is externalized by use of a surgical introducer needle. After the surgery, the rats were allowed to recover for two days in single cages under standard conditions (12 h light/dark cycle, a controlled ambient temperature of 22 ± 2°C and a relative humidity of 60 ± 10%), with free access to food and water.
In vivo microdialysis experiment
Twelve rats were randomly divided into two groups (n = 6 per group). One group of animals in the conscious condition was given the aqueous extract of Fructus Corni intra-gastricly at the dose of 1.8 g/kg for 20 min in rat, while the other group was given the aqueous extract of JZP. At the 20 min after the experiment begun, only food was withdrawn. The same dose and regime of administration was carried on. A microdialysis probe (MD-2310) was inserted into the left jugular vein. The inlet of the probe was attached to a BAS syringe driver (MD-2310, USA) connected with a controller (240V/50Hz MD-1000K, BAS, West Lafayette, IN, USA), filled with ACD solution as perfusion fluid. The vascular microdialysis probe was continuously perfused at a 2.0 μl/min flow rate. Posterior to this equilibrium period, microdialysate was collected using refrigerated fraction collector (MD-1201, BAS, West Lafayette, IN, USA) into 150 μl vials throughout the experiments. All microdialysis experiments were performed in awake freely moving animals kept in an awake animal caging system (Stand-Alone Raturn and Rodent Bowl kit, BAS, West Lafayette, IN, USA) with no anesthesia used throughout the experiment. Microdialysate samples from the blood vessel were collected for an additional 300 min automatically and stored at –20°C for centralized HPLC analysis.
At the end of each washout period, blank dialysates samples were also collected to ensure that no 5-HMF concentration was detected prior to the following administration.
RESULTS AND DISCUSSION
Safety of Fructus Corni aqueous extract
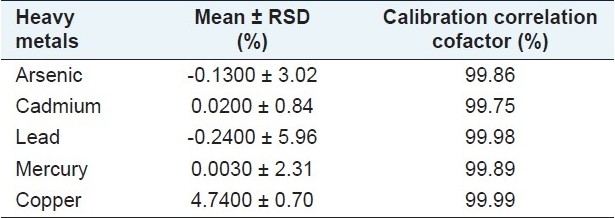
As shown in Table 1, the contents of five heavy metals (arsenic, cadmium, copper, lead, and mercury) in the aqueous extract of Fructus Corni determined by the AAS analysis fell in the range less than the maximum limit (20 ppm or μg/g) as regulated in China Pharmacopeia (2005) and the World Health Organization (WHO). The amount of the five harmful elements in the aqueous extract of Fructus Corni is in the safe range for herbal test use.
Table 1.
Content (ppm or μg/g) of five selected toxic metals in the aqueous extract of Fructus Corni
Optimization of chromatographic conditions
Different mobile phase compositions were tested: (1) aqueous–methanol; (2) aqueous–acetonitrile; (3) aqueous phosphoric acid (0.1%, v/v)–acetonitrile phosphoric acid (0.1%, v/v); (4) aqueous ammonium acetate (0.5%, v/v)–acetonitrile. As a result, the combination of aqueous phosphoric acid (0.1%, v/v)–acetonitrile for mobile phase was the best for separation. Furthermore, other chromatographic variables were also optimized including analytical columns (Hanbon Hedera ODS-2, Hanbon Lichrospher C18 and Agilent Zorbax Extend C18), the column temperatures (20°C, 25°C, and 30°C) and the flow rates (0.8 ml/min and 1.0 ml/min). Eventually, the optimal separation was achieved on an Agilent Zorbax Extend C18 column (250 mm × 4.6 mm, 5 μm) at a column temperature of 30°C with a flow rate of 1.0 ml/min.
High-performance liquid chromatographic behavior and specificity
Under the above-mentioned conditions, the retention time for 5-HMF was 9.937 min, the separation for dialysis samples was in good condition and there are no co-eluting disturbing peaks in the vicinity of the two peaks on the chromatogram of the blank dialysates. The results were shown in Figure 1.
Calibration curves, limits of detection and quantification
The calibration curves were performed with ten different concentrations in triplicate. The regression equations were calculated in the form of y = ax + b, where y and x were peak area and concentration. LOD and LOQ for each analyte were determined at a signal-to-noise ratio (S/N) of about 3 and 10, respectively. The linear regression equation (linear ranges) was y=160.38x + 0.6461(0.376–3.015 mg/L, 5-HMF). The 5-HMF in dialysate showed good linearity (r2 = 0.9999). The LOD and LOQ of the 5-HMF were 0.010 mg/l and 0.376 mg/l, respectively.
Precision and stability
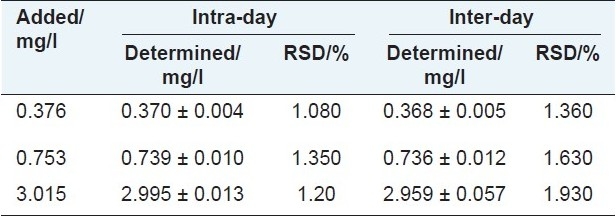
Intra-day and inter-day variations were chosen to determine the precision of the developed method by analyzing certain concentrations of standard solution (at 0.376, 0.753, and 3.015 mg/l). For intra-day variation, the standard solution was analyzed for six times within one day, the inter-day variation was determined in three consecutive days. The results were shown that in Table 2.
Table 2.
Precision of 5-HMF in freely moving rat dialysate sample. Data are expressed as mean ± S.D. (n = 5)
Stability study was performed with sample solution at room temperature and analyzed at 0 h, 2 h, 4 h, 8 h, 12 h, 24 h, and 48 h within two days, respectively. Variations were expressed by relative standard deviations (R.S.D.). The R.S.D. of stability was not more than 1.45% for all analytes.
Recovery
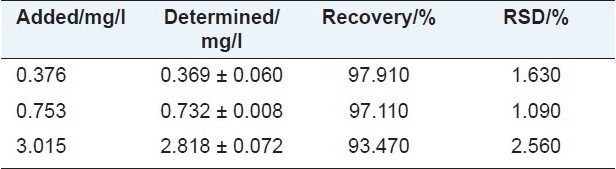
An appropriate amount of blank sample was weighed and spiked with known amount of standard solution at concentrations of 0.376, 0.753, and 3.015 mg/l. They were then treated and analyzed as described above. Each concentration was analyzed for six times. The total amount of 5-HMF was calculated from the corresponding calibration curve. The overall recoveries lay between 93.47% and 97.91% for 5-HMF with R.S.D. less than 1.36% indicating that the established method was accurate enough for the determination of the 5-HMF in SD rat microdialysate. The results were shown that in Table 3.
Table 3.
Recovery of 5-HMF in freely moving rat dialysate sample. Data are expressed as mean ± S.D. (n = 5)
Sample analysis
The established method has been successfully applied to determination the concentration of 5-HMF in crude and JZP aqueous extract from microdialysates in freely moving rats. The results showed that the concentrations of 5-HMF of crude drug aqueous extract in SD rat microdialysate, JZP aqueous extract varied significantly. For instance, for the crude Fructus Corni aqueous extract, the concentration of 5-HMF in microdialysate was 1.4951 μg/l, but higher in rat microdialysate after i.g. administration of the aqueous extract of JZP (5.2662 μg/l). The variations might result from processing procedures for Fructus Corni. It was reported that processing or heating could drastically increase the content of 5-HMF.
CONCLUSION
Microdialysis sampling technique causing minimal perturbation to physiological processes has been to date extensively used in and greatly contributory to pharmacodynamics, drug disposition, and metabolism researches in which microdialysis sampling is especially of significance in that traditional sampling methods exhibit evidently more disadvantages. Microdialysis technique with exclusive characteristics of high temporal and spatial resolutions is quite suitable for drug delivery research, specifically in the blood. Additionally, microdialysis also makes it feasible to perform real time and on-line experiment on a free lying animal. The study by Mayor and Illum suggested that the use of anesthetics was proposed to a variable extent to attenuate the ability of the gastrointestinal tract clearance.[16] As a result, a prolonged residence time of gastrointestinal tract formulation in the gastrointestinal tract was obtained.
In this paper, a freely moving rat model with no anesthesia introduced was utilized throughout the experiment in order to deplete the influence of anesthesia on absorption via gastrointestinal tract with access to physiological condition. The application of microdialysis technique and a free lying animal model contributed to employing a concentration experimental design which not only reduced the amount of experimental animal used and experimental cost, but minimum the influence of anatomical and physical differences between individual animals on experimental data.
This valuable information concerning the concentrations of 5-HMF in freely moving rats after i.g. administration of the aqueous extract of crude drug and JZP which can be of great importance for pharmaceutical industry. Furthermore, from the comparison between the contents in a free lying rats after i.g. administration of the crude and processed Fructus Corni, it can be obviously seen that the contents are enormously changed before and after being processed; this preliminarily explains the reason why crude and processed Fructus Corni aqueous extract have traditionally been used for treating different clinical symptoms and expatiates upon the process principium.
However, further studies involving complete characterization and bioassay analysis of 5-HMF in the aqueous extract of crude drug and JZP are necessary. There is ongoing research in this direction in our laboratory which will be reported in a future manuscript. In a word, the method demonstrates a relatively short analysis time, acceptable sensitivity, precision, accuracy, selectivity, recovery, and stability. And to our knowledge, it is in fact the first report of microdialysis sampling coupled with HPLC-DAD for in vivo determination and study of the concentrations of 5-HMF in free lying rats after i.g. administration of the aqueous extract of crude and processed Fructus Corni and it is benefit to have a further exploration on the mechanism of 5-HMF in treatment.
ACKNOWLEDGEMENTS
The authors would also like to express special thanks to Mr.Jiao Kun and Mr. Zhuo tao for their technical support.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Wen XD, Qi LW, Chen J, Song Y, Yi L, Yang XW, et al. Analysis of interaction property of bioactive components in Danggui Buxue Decoction with protein by microdialysis coupled with HPLC-DAD-MS. J Chromatogr B. 2007;852:598–604. doi: 10.1016/j.jchromb.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 2.Qian ZM, Qin SJ, Yi L, Li P, Wen XD. Binding study of Flos Lonicerae Japonicae with bovine serum albumi-n using centrifugal ultrafiltration and liquid chromatography. Biomed Chromatogr. 2008;22:202–6. doi: 10.1002/bmc.916. [DOI] [PubMed] [Google Scholar]
- 3.Deterding LJ, Dix K, Burka LT, Tomer KB. On-line coupling of in vivo microdialysis with tandem masss pec-trometry. Anal Chem. 1992;64:2636–41. doi: 10.1021/ac00045a029. [DOI] [PubMed] [Google Scholar]
- 4.Bellander BM, Cantais E, Enblad P. Consensus meeting on MD in neurointensive care. Intensive Care Med. 2004;30:2166–99. doi: 10.1007/s00134-004-2461-8. [DOI] [PubMed] [Google Scholar]
- 5.Ding X, Wang MY, Yu ZL, Hu W, Cai BC. Studies on separation, appraisal and the biological activity of 5-HMF in Cornus officinalis. (484).Zhongguo Zhong Yao Za Zhi. 2008;33:392–6. [PubMed] [Google Scholar]
- 6.Du WF, Cai H, Wang MY, Ding X, Yang H, Cai BC. Simultaneous determination of six active components in crude and processed Fructus Corni by high performance liquid chromatography. J Pharm Biomed Anal. 2008;48:194–7. doi: 10.1016/j.jpba.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Xu Q, Li YH, Lü XY. Investigation on influencing factors of 5-HMF content in Schisandra. J Zhejiang Univ Sci B. 2007;8:439–45. doi: 10.1631/jzus.2007.B0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li YH, Lu XY. Investigation on the origin of 5-HMF in Shengmaiyin decoction by RP-HPLC method. J Zhejiang Univ Sci B. 2005;6:1015–21. doi: 10.1631/jzus.2005.B1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brustugun J, Tønnesen HH, Edge R, Navaratnam S. Formation and reactivity of free radicals in 5-hydroxymethyl-2-furaldehyde–the effect on isoprenaline photostability. J Photochem Photobiol B. 2005;79:109–19. doi: 10.1016/j.jphotobiol.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Graff CL, Pollack GM. Nasal drug administration: Potential for targeted central nervous system delivery. J Pharm Sci. 2005;94:1187–95. doi: 10.1002/jps.20318. [DOI] [PubMed] [Google Scholar]
- 11.Hatano T, Yasuhara T, Abe R, Kudat TO. A galloylated monoterpene glucoside and a dimeric hydrolyzable tannin from Cornus officinalis. Phytochemistry. 1990;29:2975–8. [Google Scholar]
- 12.Illum L. Nasal drug delivery: Possibilities, problems and solutions. J Control Release. 2003;87:187–98. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 13.Mayor SH, Illum L. Investigation of the effect of anaesthesia on nasal absorption of insulin in rats. Int J Pharm. 1997;149:123–9. [Google Scholar]
- 14.De Lange EC, De Boer AG, Breimer DD. Methodological issues in microdialysis sampling for pharmacokinetic studies. Adv Drug Deliv Rev. 2000;45:125–48. doi: 10.1016/s0169-409x(00)00107-1. [DOI] [PubMed] [Google Scholar]
- 15.Tsai TH. Assaying protein unbound drugs using microdialysis techniques. J Chromatogr B. 2003;797:161–73. doi: 10.1016/j.jchromb.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Davies MI. A review of microdialysis sampling for pharmacokinetic applications. Anal Chim Acta. 1999;379:227–49. [Google Scholar]


