Abstract
Identification of metastatic melanoma can be difficult because of its great morphological variation and mimicry of a wide variety of other tumors. The more melanoma specific melanoma markers: melanA/MART-1, HMB45, and tyrosinase, used in addition to S100 protein, each have limitations in sensitivity and specicifity. In this study, we evaluated two newer melanoma markers: monoclonal antibodies KBA62 and PNL2 to yet unidentified antigens, using a large panel of metastatic melanomas (n=214), desmoplastic melanomas (n=34), GI mucosal melanomas (n = 54), benign nevi (n=27), clear cell sarcomas (n = 16) and non-melanocytic tumors (n=1218). Immunoreactivity for KBA62 and PNL2 was found in all pigmented nevi and in 86% and 90% of metastatic melanomas, respectively. Mucosal melanomas showed a similar rate of PNL2 immunoreactivity, but somewhat less frequent KBA62-positivity (72%). In addition, KBA62 was found to be a sensitive diagnostic marker for desmoplastic melanoma (28/34; 82%), whereas PNL2 was only rarely positive (2/34; 6%). KBA62 positive normal tissues included pericytes, vascular and parenchymal smooth muscle, basal cells of complex epithelia, including myoepithelia, while PNL2 labeled only melanocytes and neutrophils. Among non-melanocytic tumors, KBA62-positive were nodular fasciitis, leiomyoma, and leiomyosarcoma, gastrointestinal stromal tumor, benign and malignant nerve sheath tumors, synovial sarcoma, and subsets of various carcinomas, especially those with squamous cell/stratified epithelial differentiation. PNL2-positivity in non-melanocytic tumors was more restricted but occurred consistently in angiomyolipoma and other PEComas, and in chronic myeloid leukemia tissue infiltrates. KBA62 may assist in identification of desmoplastic melanomas, but its widespread occurrence in non-melanomas limits utility. PNL2 is highly specific for melanomas, but lacks reactivity with desmoplastic melanomas. It is also an excellent supplementary marker for PEComas at various sites.
Keywords: KBA62, PNL2, metastatic and desmoplastic melanoma, PEComa, immunohistochemistry
INTRODUCTION
The often challenging histologic and immunohistochemical differential diagnosis of metastatic malignant melanoma frequently requires application of more than two or three melanoma markers. All the current main markers have some shortcomings. Even the first line marker, S100 protein, is not expressed in all melanomas. Being a marker related to melanogenesis, gp100 (detected by the HMB45 monoclonal antibody) fails to react with many amelanotic tumors, and immunoreactivity for MelanA/Mart-1 and tyrosinase is likewise absent in 10–15% of metastatic melanomas. On the other hand, a sensitive marker, microphthalmia transcription factor, has many isoforms expressed in other tissues so that a positive result does not necessarily reflect melanoma.3,5
Two relatively new melanoma markers, monoclonal antibodies KBA62 and PNL2 to yet unidentified antigens were investigated in this study using a large panel of metastatic melanomas, primary desmoplastic melanomas, as well as panels of normal tissue and non-melanocytic tumors.
KBA62, raised to the KAL melanoma cell line, recognizes an unknown determinant expressed in melanoma cells and commonly malignant melanoma2,3,8. This antibody reacted with protein bands of 140, 135, 128, 88, and 73 kD in immunoblotting. It was further evaluated in one study, which found immunoreactivity in most metastatic melanomas, desmoplastic melanomas, and some carcinomas, especially well-differentiated squamous ones.3
The PNL2 monoclonal antibody raised to human somatostatin receptor was unexpectedly found to strongly label melanocytes and melanomas, instead of the intended target protein.6,9 It was further examined in one study.1 Non-melanocytic lesions found to be positive with this marker include PEComa family tumors.1,4,9
In this study, we examined metastatic, mucosal, and desmoplastic melanomas, and a broad range of normal and neoplastic tissues other than melanomas to obtain a more complete understanding on the potential of these antibodies in surgical pathology, especially in the differential diagnosis of malignant melanoma.
MATERIALS AND METHODS
A selection of normal tissues, melanocytic neoplasms (n = 345), and a wide variety of non-melanocytic neoplasms (n = 1218) were immunohistochemically examined using monoclonal antibodies KBA62 and PNL2. There were 214 metastatic melanomas, 34 desmoplastic melanomas, 54 primary gastrointestinal mucosal melanomas, 16 clear cell sarcomas, and 27 cutaneous pigmented nevi.
The non-melanocytic tumors included 785 carcinomas, 383 mesenchymal and neuroectodermal tumors, and 50 hematopoietic tumors. Most test slides were derived from multitumor blocks containing 5–50 cases. These blocks were assembled manually by embedding pieces on tumors in liquid paraffin in 5–7 rows, 5–10 pieces in each row.
The primary antibodies, KBA62 and PNL2, were obtained from Cell Marque (Rocklin, CA), and cytokeratin 5/6 (clone D5/16B4) from DAKO Cytomation (Carpinteria, CA) and each diluted 1:100. Immunostaining was performed with Leica Bond-Max automatic immunostainer (Bannockburn, IL). Heat induced epitope retrieval (high pH, EDTA-based buffer, pH 9.0, Leica Bond-Max) was applied for 25 minutes. The primary antibody was incubated for 30 minutes at room temperature. Leica Bond-Max avidin-biotin free polymer system was used in the detection. Diaminobenzidine was used as the chromogen, following the blocking of endogenous peroxidase with 3% hydrogen peroxide diluted in phosphate buffer. Immunostained slides were counterstained with hematoxylin. The results from additional immunostainings used for comparison; S100, HMB45, Melan A, Tyrosinase, and MITF were from a previous study.5
Immunoreactivity in tumor cells was semiquantitatively assessed: <10% tumor cells positive: 1+; 10–30% of tumor cells positive: 2+; >30% of tumor cells positive: 3+.
RESULTS
Normal tissues
Strong KBA62 immunoreactivity was detected in vascular pericytes and smooth muscle but not in endothelia. Both vascular and visceral smooth muscle (gastrointestinal tract, gallbladder, and prostate) showed positivity with a membranous staining pattern. Mesangium of kidney glomeruli, subset of breast myoepithelia and basal cells of epidermis, tonsil, and uterine exocervix, hair shaft epithelia of skin, some subepithelial stromal cells especially of urinary bladder, and astrocytes were positive. Bronchial cartilage cells were variably positive.
PNL2-immunoreactivity (cytoplasmic, often granular staining pattern) was restricted to melanocytes, granulocytes, and osteoclastic giant cells.
Melanocytic neoplasms
KBA62 and PNL2 immunoreactivity in melanocytic neoplasms is summarized in Table 1. Nearly all nevi show prominent cytoplasmic KBA62 and PNL2-immunoreactivity (Table 1). KBA62-positivity was detected in 86.3% and PNL2-positivity in 89.2% of metastatic melanomas. Nearly 80% of the positive cases showed immunoreactivity in >30% of tumor cells for both markers (Fig1A and 2A). However, only focal immunoreactivity in < 10% of tumor cells was seen in 5.4% of cases for KBA62 and in 12.0% for PNL2.
Table 1.
Summary of KBA62 and PNL2 immunoreactivity in melanocytic neoplasms.
| Results on KBA62 | Positive % | Negative | >0–<10% | 10–30% | >30% |
|---|---|---|---|---|---|
| Metastatic malignant melanomas | 184/214 86% | 30 | 10 | 26 | 148 |
| PNL2-negative | 18/24 75% | 6 | 0 | 0 | 18 |
| S100-negative | 5/10 50% | 5 | 0 | 1 | 4 |
| HMB45-negative | 24/30 80% | 6 | 2 | 5 | 17 |
| Melan A-negative | 19/26 73% | 7 | 0 | 2 | 17 |
| Tyrosinase-negative | 8/14 57% | 6 | 0 | 1 | 7 |
| MITF-negative | 21/27 78% | 6 | 0 | 4 | 17 |
| Negative for all of above except S100 | 8/10 80% | 2 | 0 | 1 | 7 |
| Desmoplastic melanoma | 28/34 82% | 5 | 0 | 5 | 23 |
| Mucosal melanoma (GI) | 39/54 72% | 15 | 2 | 13 | 24 |
| Clear cell sarcoma | 15/16 94% | 1 | 4 | 4 | 7 |
| Pigmented nevi | 27/27 100% | 0 | 0 | 0 | 0 |
| Results on PNL2 | |||||
| Metastatic malignant melanomas | 190/214 (89%) | 24 | 23 | 23 | 144 |
| KBA62-negative | 25/30 83% | 5 | 2 | 0 | 23 |
| S100-negative | 7/10 70% | 3 | 2 | 0 | 5 |
| HMB45 -negative | 7/28 25% | 21 | 6 | 0 | 1 |
| Melan A-negative | 12/27 44% | 15 | 4 | 1 | 7 |
| Tyrosinase-negative | 4/16 25% | 12 | 2 | 0 | 2 |
| MITF-negative | 16/31 52% | 15 | 5 | 0 | 11 |
| Negative for all of above except S100 | 1/10 10% | 9 | 1 | 0 | 0 |
| Desmoplastic melanoma | 2/34 6% | 28 | 0 | 0 | 0 |
| Mucosal melanoma (GI) | 48/54 89% | 6 | 2 | 7 | 39 |
| Clear cell sarcoma | 16/16 100% | 0 | 1 | 1 | 14 |
| Pigmented nevi | 25/27 93% | 2 | 0 | 0 | 25 |
| Total | 345 | ||||
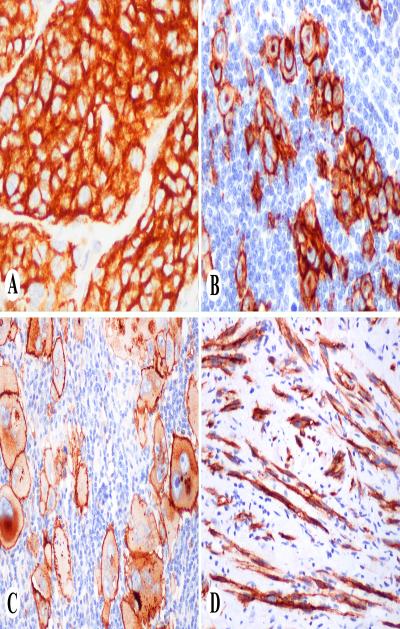
Fig. 1.
KBA62 in melanocytic neoplasms. A. Clusters of nevus cells show strong cytoplasmic positivity. B. Nodal melanoma metastasis shows membranous, cytoplasmic and Golgi-zone staining pattern. C. Pleomorphic metastatic melanoma shows prominent membrane and variable cytoplasmic and golgi-zone staining. D. Bundles of desmoplastic melanoma cells are KBA62-positive.
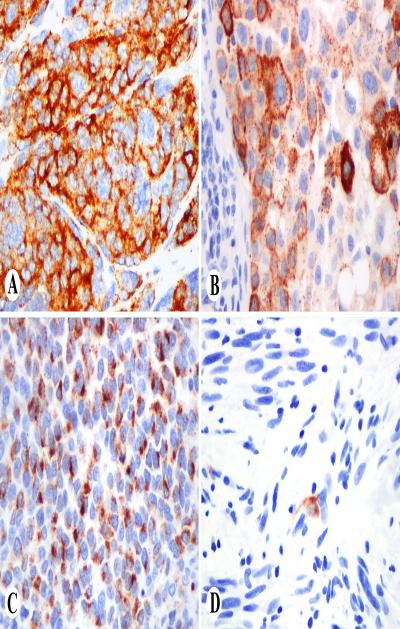
Fig. 2.
PNL2 in melanocytic neoplasms. A. clusters of nevus cells show strong cytoplasmic positivity. B. Nodal melanoma metastasis shows granular cytoplasmic positivity. C. Metastatic melanoma with prominent Golgi-zone positivity. D. Only isolated cells are positive in desmoplastic melanoma.
In metastatic melanomas, KBA62 immunostaining varied from diffuse cytoplasmic to membranous, with some cases also displaying a Golgi-zone pattern (Fig. 1B and C). PNL2-immunoreactivity was usually diffused cytoplasmic, often with a granular pattern, but some cases showed a prominent golgi-zone pattern (Fig. 2B and C).
Mucosal melanomas of esophagus and anorectum (great majority of them primary) showed KBA-positivity is 29/54 cases (72%), and PNL2-positivity in 48/54 cases (89%).
Desmoplastic melanomas (n = 34) were positive for KBA62 in 82.4% of cases, usually with the majority of tumor cells being positive (Fig 1D). However, only 2 desmoplastic melanomas were positive for PNL2 containing only occasional positive tumor cells (Fig. 2D). In mucosal melanomas (n=54), 72% and 89% of the cases were positive for KBA62 and PNL2 respectively.
Most clear cell sarcomas (15/16) contained KBA62 and PNL2-positive tumor cells, typically a majority of tumor cells.
Malignant epithelial tumors
KBA62 and PNL2-immunoreactivity in 785 carcinomas and mesotheliomas are summarized in Table 2 and illustrated in Fig 3D.
Table 2.
Summary of KBA62 and PNL2 immunreactivity in malignant epithelial neoplasms.
| Tumor type | KBA62 | PNL2 |
|---|---|---|
| Bladder, transitional cell carcinoma | 3/25 | 0/25 |
| Breast, ductal carcinoma | 9/66 | 0/66 |
| Breast, lobular carcinoma | 0/5 | 0/5 |
| Colon, adenocarcinoma | 0/48 | 0/48 |
| Endometrium, adenocarcinoma | 2/20 | 0/20 |
| Liver, cholangiocarcinoma | 4/20 | 0/20 |
| Liver, hepatocellular carcinoma | 0/39 | 0/39 |
| Lung, adenocarcinoma | 4/32 | 0/32 |
| Lung, large cell undifferentiated carcinoma | 5/45 | 0/45 |
| Malignant mesothelioma* | 5/31 | 0/31 |
| Merkel cell carcinoma | 0/4 | 0/4 |
| Ovary, adenocarcinoma (mostly serous) | 3/34 | 0/34 |
| Pancreas, adenocarcinoma | 5/27 | 0/27 |
| Prostate, adenocarcinoma | 0/50 | 0/50 |
| Stomach, adenocarcinoma | 2/48 | 0/48 |
| Sarcomatoid carcinoma, intestine/larynx | 18/39 | 0/39 |
| Squamous cell carcinoma, larynx | 26/31 | 0/31 |
| Squamous cell carcinoma, cervix | 14/22 | 0/22 |
| Squamous cell carcinoma, esophagus | 17/27 | 0/25 |
| Squamous cell carcinoma, lung | 25/54 | 0/54 |
| Testis, embryonal carcinoma | 2/22 | 0/22 |
| Testis, seminoma* | 12/43 | 0/43 |
| Thyroid, follicular carcinoma | 2/4 | 0/4 |
| Thyroid, papillary carcinoma | 1/49 | 0/49 |
| Total | 159/785 | 0/783 |
Delicate membrane staining
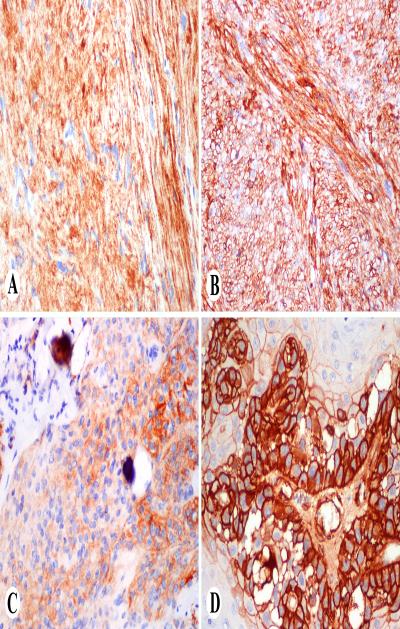
Fig. 3.
Examples of KBA62-positive non-melanocytic neoplasms. A. Schwannoma. B. Leiomyosarcoma. C. Meningioma. D. Squamous cell carcinoma.
KBA62-positivity was variably seen in squamous cell carcinomas of different sites, most frequently in laryngeal ones. In addition, almost half of sarcomatoid carcinomas of larynx and intestine, many breast carcinomas with basal cell/myoepithelial differentiation (cytokeratin 5/6 positive) and a papillary thyroid carcinoma with focal squamoid differentiation (cytokeratin 5/6 positive) were KBA62-positive.
Adenocarcinomas had a relatively low rate of KBA62-positivity. Among adenocarcinomas, 8.8% of ovarian, 12.5% of pulmonary, 10% of endometrial, 4.2% of gastric, 18.5% of pancreatic, and none of prostatic and colonic adenocarcinomas contained KBA62 immunoreactivity with all the positive cases. Among hepatic tumors, KBA62 positivity was detected in some cholangiocarcinomas but in none of the hepatocellular carcinomas.
Delicate membrane positivity for KBA62 was detected in 5/31 mesotheliomas and 12/43 testicular seminomas.
PNL2-immunoreactivity was not encountered in any carcinomas other than necrotic areas containing intact or disintegrating neutrophils present in 51 of 785 carcinomas of different types, especially including embryonal carcinoma of testis (24%), squamous cell carcinoma of lung (26%), and adenocarcinomas of endometrium (26%), colon (11%), stomach (10%), and ovary (8.1%).
Mesenchymal and Neuroectodermal Malignant Tumors
PNL2 and KBA62-immunoreactivity in 383 non-melanocytic mesenchymal and neuroectodermal lesions is summarized in Table 3.
Table 3.
Summary of KBA62 and PNL2 immunoreactivity in mesenchymal, neuroectodermal and hematopoietic neoplasms.
| KBA62 | PNL2 | |
|---|---|---|
| Mesenchymal and neuroectodermal tumors | ||
| PEComa, liver and uterus | 1/13 | 15/16 |
| Lymphangioleiomyoma | 3/7 | 7/7 |
| Angiomyolipoma, kidney | 0/17 | 14/17 |
| Angiosarcoma | 4/14 | 0/14 |
| GISTs of stomach and small intestine | 73/103 | 0/103 |
| Hemangiopericytoma/Solitary fibrous tumor | 10/36 | 0/36 |
| Leiomyosarcoma | 32/33 | 0/33 |
| Malignant fibrous histiocytoma | 11/32 | 0/32 |
| Malignant peripheral nerve sheath tumor | 16/21 | 0/21 |
| Nodular fasciitis | 15/15 | 0/15 |
| Meningioma | 4/16 | 0/16 |
| Neurofibroma (different variants) | 42/42 | 42/42 |
| Schwannoma | 13/17 | 0/17 |
| Synovial sarcoma | 9/14 | 0/14 |
| Hematopoietic neoplasms | ||
| Diffuse large B cell lymphoma | 0/20 | 0/20 |
| Small B-cell lymphomas | 0/13 | 0/13 |
| Chronic myeloid leukemia (spleen) | 1/6 | 6/6 |
| Blastic extramedullary myeloid tumor | 1/11 | 0/11 |
| Total | 235/430 | 42/433 |
GIST = Gastrointestinal stromal tumor
High KBA62 protein expression was seen in sarcomas and benign mesenchymal tumors of different phenotypes including nerve sheath, myofibroblastic, and smooth muscle tumors, gastrointestinal stromal tumors (GISTs), and undifferentiated sarcomas, each of which were positive in a majority of cases (Fig 3).
The majority of GISTs (73/103), nodular fasciitis (15/15), neurofibromas including cutaneous, diffuse, and intraneural examples (42/42), schwannomas (13/17) (Fig 3A), malignant peripheral nerve sheath tumors (MPNSTs) (16/21), leiomyosarcomas (32/33) (Fig 3B), synovial sarcomas (9/14), lymphangioleiomyomas (3/7), malignant fibrous histiocytomas (11/32), angiosarcomas (4/14), hemangiopericytomas (10/36), and meningiomas (4/16) (Fig 3C) were KBA62 positive.
PNL2 expression was detected only in perivascular epitheloid cell tumor (PEComa) family of tumors. Varying numbers of PNL2 positive epithelioid cells were detected in 14/17 angiomyolipomas of kidney (Fig.4A and B). All seven lymphangiomyomas of the retroperitoneum, 12/13 PEComas of the uterus (Fig.4C), 2/2 PEComas of the liver, and 1 gastric PEComa contained large numbers of PNL2-positive cells. These PEComas were highlighted as the sole PNL2 positive tumors in multitumor blocks containing hepatocellular carcinomas, uterine smooth muscle tumors, and GISTs, respectively.
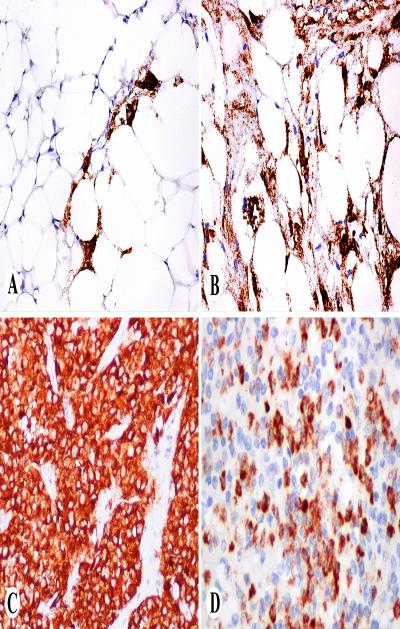
Fig. 4.
Examples of PNL2 in non-melanocytic neoplasms. A, B. Angiomyolipomas with scattered positive cells and extensive positivity. C. Uterine PEComa with strong cytoplasmic positivity. D. Chronic myeloid leukemia infiltration in spleen shows PNL2-positive myeloid cells.
Hematopoietic neoplasms
Among 50 hematopoietic neoplasms examined, all lymphomas (n=33) were negative for KBA62 and PNL2. PNL2 expression was detected in subsets of neutrophils and myelocytes in all six chronic myeloid leukemia tissue infiltrates (Fig 4D), whereas all blastic extramedullary myeloid tumors were negative. In most cases, KBA62 highlighted vascular pericytes as the sole positive cells. One case each of acute and chronic myeloid leukemia contained KBA62-positive cells (Table 3).
DISCUSSION
In this study, we examined large numbers of metastatic melanomas and other melanocytic neoplasms and their mimics for two relatively new melanoma markers: monoclonal antibodies designated KBA62 and PNL2.
The antigens detected by KBA62 and PNL2 remain unknown. KBA62 is a monoclonal antibody raised to a melanoma cell line recognizing yet an unidentified epitope.2 PNL2 monoclonal antibody was raised to somatostatin receptor type 2,9 but found non-reactive to the intended target protein.
Immunostaining patterns of KBA62 and PNL2 are dissimilar to all previously commonly used antibodies: S100 protein, HMB45, MelanA/Mart-1, tyrosinase, and microphthalmia transcription factor. KBA62 also detects pericytes, unlike any other melanoma antibodies. PNL2 also reacts with neutrophils, although the granular immunoreactivity and positivity in PEComas resemble the features of HMB45.
KBA62 and PNL2 were sensitive markers for metastatic melanoma being expressed in a great majority of cases: overall 86% and 89%, respectively. Primary mucosal melanomas showed approximately similar frequencies of KBA62- and PNL2-positivity. Moreover, KBA62 and PNL2 also recognized S100 protein negative metastatic melanomas (KBA62: 4/7 and PNL2: 6/7) indicating that these new markers are useful diagnostic complements for this rare subgroup (2% of all metastatic melanomas). They also identified some melanomas negative for the other older melanoma markers: HMB45, MelanA, tyrosinase, and microphthalmia transcription factor. PNL2 seems superior to KBA62 in the detection of mucosal melanomas.
Desmoplastic melanoma can be a difficult diagnostic problem. Due to its dispersed and infiltrative nature, it can be difficult to identify as a malignancy, especially in small biopsies. Together with S100 protein, KBA62 may help to highlight desmoplastic melanoma cells from fibroblastic or non-specific mesenchymal components, whereas neither marker helps to separate desmoplastic melanoma from nerve sheath tumors (both are KBA62 and S100-protein positive). In detection of desmoplastic melanomas, KBA62 is clearly superior to PNL2, which generally fails to detect desmoplastic melanoma cells. In this respect, PNL2 is similar to older melanoma markers other than S100 protein (HMB45, MelanA, tyrosinase, microphthalmia transcription factor) which generally do not detect desmoplastic melanomas.5 Ultimately, desmoplastic melanoma has to be diagnosed based on combination of morphology and immunohistochemistry: atypical to focally pleomorphic, variably bundled spindle-shaped, S100 protein and KBA62 positive dermal/subcutaneous cells, often associated with spotty lymphoid infiltration, and a conventional melanoma component in primary cutaneous lesions.
The neutrophilic expression of PNL2 is reflected as PNL2-positivity of differentiated myeloid neoplasms (chronic myelogenous leukemia), whereas acute myeloid leukemia cells do not retain PNL2-positivity, as shown in one previous study.1 Another probable consequence of neutrophilic expression of PNL2 is positivity in tumor necrosis and their borders.7 This immunoreactivity should not be mistaken for melanocytic differentiation.
PEComa family tumors (myomelanocytic tumors) are mesenchymal tumors notable for their positivity for HMB45 and variable coexpression of smooth muscle markers, especially smooth muscle actin. They include angiomyolipoma of kidney (and abdomen), lymphangiomyoma(tosis), and rare organ-based tumors, generally designated as PEComas. In this study, we found a great majority of PEComas to be PNL2-positive. As PNL2-positivity among mesenchymal non-melanocytic tumors is very specific for PEComa, PNL2 is a useful supplemental marker for PEComa, in addition to HMB45. Our results confirm previous findings on a smaller number of PEComas.1,4 However, PEComas were generally negative for KBA62, although otherwise, this marker is fairly extensively expressed in other mesenchymal neoplasms.
KBA62 positivity was in addition to melanocytic neoplasms also commonly detected in a wide variety of mesenchymal and epithelial neoplasms. Its common immunoreactivity in smooth muscle and related tumors is not unexpected, as various normal vascular and parenchymal smooth muscle elements and pericytes are also KBA62-positive (not endothelia cells, as positivity was previously ascribed to8). Similarly, myofibroblastic neoplasms and GISTs were also commonly positive, as were some angiosarcomas. Schwannian tumors were also positive, which indicates that KBA62 is not useful in the differential diagnosis between melanoma and nerve sheath tumors.
In addition, fairly common and sometimes prominent KBA62-immunoreactivity in carcinomas of various types including differentiated and undifferentiated forms, illustrates serious limitations of the melanocytic specificity of KBA62. It is very clear that KBA62 has to be used as a component of a panel addressing the diagnosis of epithelial and mesenchymal tumors. Perhaps the greatest potential value of KBA62 lies in its ability to detect desmoplastic melanomas in conjunction with S100-protein, whereas most melanoma markers other than S100 protein fail in this.
Interestingly, in our study we found that in addition to prominent expression in most of the squamous cell carcinoma examined, KBA62-positivity was also seen in many breast carcinomas with complex epithelial differentiation and a papillary carcinoma of thyroid with a portion of squamoid differentiation confirmed with cytokeratin 5/6 immunostain. This finding suggests that there may be a correlation between KBA62 expression and squamoid or basal/myoepithelial cell differentiation of the tumor, and therefore KBA62 might be a useful adjunct marker for this morphologically and clinically distinct subset of carcinomas, especially those of breast.
To our knowledge, we present hitherto largest study on two relatively new melanoma markers, PNL2 and KBA62, in benign and malignant melanoma and other non melanocytic malignant neoplasms. Although both PNL2 and KBA62 positivity was demonstrated in more than 85% of epitheloid melanocytic lesions, we confirmed that immunoreactivity of PNL2 is restricted to melanocytes with the exception of necrotic ghost tumor cells, PEComa family tumors, and chronic myeloid leukemia. This high specificity of PNL2 for melanocytes makes this marker a useful additional tool for the diagnosis of non-desmoplastic melanoma. Our study also supports KBA62 is a potential marker for diagnosis of desmoplastic melanomas. Moreover, these two markers, KBA62 and PNL2, are useful complements for the detection of melanomas negative for S100 protein, HMB45, melanA, and other supplementary melanoma markers.
Acknowledgments
Supported by the NIH/NCI Intramural Research Program.
Footnotes
None of the authors have a conflict of interest or funding to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Busam KJ, Kucukgol D, Sato E, et al. Immunohistochemical analysis of novel monoclonal antibody PNL2 and comparison with other melanocyte differentiation markers. Am J Surg pathol. 2005;29:400–406. doi: 10.1097/01.pas.0000152137.81771.5b. [DOI] [PubMed] [Google Scholar]
- 2.Cohen-Knafo E, Al-Saati T, Aziza J, et al. Production and characterization of an antimelanoma monoclonal antibody KBA.62 using a new melanoma cell line reactive on paraffin wax embedded sections. J Clin Pathol. 1995;48:826–831. doi: 10.1136/jcp.48.9.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufmann O, Koch S, Burghardt J, et al. Tyrosinase, Melan-A, and KBA 62 as markers for the immunohistochemical identification of metastatic amelanotic melanomas on paraffin sections. Mod Pathol. 1998;11:740–746. [PubMed] [Google Scholar]
- 4.Horn LC, Einenkel J. Uterine perivascular epitheloid cell tumor (PEComa) with CD117 and PNL2 positivity and entrapped endometriotic glands, mimicking sex-cordlike differentiation. Ann Diagn Pathol. 2011;15:216–8. doi: 10.1016/j.anndiagpath.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Miettinen M, Fernandez M, Franssila K, et al. Microphthalmia transcription factor in the immunohistochemical diagnosis of metastatic melanoma - comparison with four other melanoma markers. Am J Surg Pathol. 2001;25:205–211. doi: 10.1097/00000478-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Morris LG, Wen YH, Nonaka D, et al. PNL2 melanocytic marker in immunohistochemical ebaluation of primary mucosal melanoma of the head and neck. Head and Neck. 2008;30:771–775. doi: 10.1002/hed.20785. [DOI] [PubMed] [Google Scholar]
- 7.Nonaka D, Laser J, Tucker R, et al. Immunohistochemical evaluation of necrotic malignant melanomas. Am J Clin Pathol. 2007;127:787–791. doi: 10.1309/WKEN4ER9GXJ9GG31. [DOI] [PubMed] [Google Scholar]
- 8.Pages C, Pochaic P, al Saati T, et al. KBA.62: a useful marker for primary and metastatic melanomas. Hum Pathol. 2008;39:1136–1142. doi: 10.1016/j.humpath.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Rochaix P, Lacroix-Triki M, Lamant L, et al. PNL2, a new monoclonal antibody directed against a fixative-resistant melanocyte antigen. Mod Pathol. 2003;16:481–490. doi: 10.1097/01.MP.0000067686.34489.50. [DOI] [PubMed] [Google Scholar]