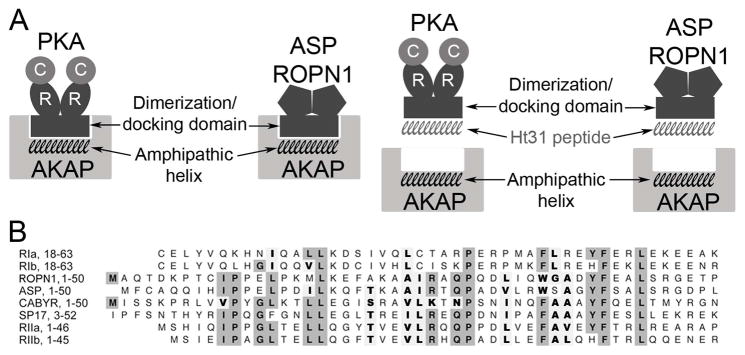
Figure 1. Model of AKAP interaction with PKA RII and RII-like (R2D2) proteins.
In A, the amphipathic helix region on AKAPs interacts with the dimerization/docking (R2D2) domain of both PKA and R2D2 proteins ASP and ROPN1. This interaction is interrupted by Ht31 competitively binding to the R2D2 domains of PKA and the R2D2 proteins. In B, amino acid sequence alignment of the R2D2 domains of ROPN1, ASP, SP17, CABYR, PKA RI and RII. Gray boxes highlight conserved identity, bold letters highlight conserved similarity.