Abstract
Stem cells represent an important tool in veterinary therapeutic field such as tissue engineering. In the present study, equine amnion-derived mesenchymal stromal cells were investigated for applications in veterinary science as an alternative source to bone marrow mesenchymal stem cells and adipose stem cells. Amnion stromal cells isolation and characterization protocol is described; the in vitro cell growth rate was calculated by measuring viable cell number over 20 days. The expression of stem cell markers such as Oct-4, Nanog, Sox-2 and CD105 was assessed by retrotranscription quantitative PCR (RT-qPCR) and differentiation into adipocytes, osteocytes and chondrocytes precursors was analyzed by cytochemical staining. This study showed that amnion stromal cells expressing stem cell markers can differentiate into mesoderm lineage and may be an alternative source to mesenchymal stem cells derived from adipose tissue and bone marrow for the use in tissue repair.
Keywords: Equine, Amnion, Gene expression, Differentiation
Introduction
In recent times embryonic and adult stem cells have aroused increased interest due to research studies in developmental biology and their use in regenerative medicine. Stem cells are an interesting cellular model to study differentiation mechanisms because of the relative ease of establishing in vitro cultures and their good proliferation (Bianco et al. 2001). Equine mesenchymal stem cells have been established in vitro from different tissues such as bone marrow (Fortier et al. 1998), umbilical cord blood (Reed and Johnson 2008) and adipose tissue (Vidal et al. 2007) and show differentiation capabilities into mesodermal lineages. Nevertheless, alternative cell sources to be used in veterinary clinical applications such as tissue engineering and repair are still necessary. In this regard amnion may represent a rich source of undifferentiated cells. The amnion is composed of an epithelial layer arising from embryonic epiblast cells prior to gastrulation and an outer layer of connective tissue contiguous with the fetal skin. It has been reported that rat and human mesenchymal stromal cells can be isolated from amnion (Ilancheran et al. 2009). These cell populations display a fibroblast like appearance, adhere onto plastic culture vessels, form clonal colonies (Kim et al. 2007) and can differentiate into mature cell lineages in vitro with ectodermal and endodermal characteristics (Marcus et al. 2008; Parolini et al. 2008), thus resulting a potential source for regenerative medicine applications.
Specific transcription factors define mesenchymal stromal characteristics involved in maintaining the undifferentiated stem cell pattern required for self-renewal (Greco et al. 2007) and are expressed by different animal tissue-derived mesenchymal stromal cells such as horse bone marrow stem cells (Violini et al. 2009) and rat amniotic membrane stem cells (Marcus et al. 2008); Moreover human amnion stromal cells were reported to express cell surface markers such as octamer-binding transcription factor Oct4, Nanog (Toda et al. 2007), CD105 but not hematopoietic markers such as CD34 (In’t Anker et al. 2004; Parolini et al. 2008).
The present study describes a protocol to isolate an equine amnion stromal cell line and its characterization by differentiating into mesodermal lineage precursors.
Materials and methods
Amnion stromal cell line isolation and growth
Within 40 min after parturition the amnion stromal membrane from an 8-year-old thoroughbred mare was carefully peeled off from the rest of chorion layer. The membrane was washed in Phosphate Buffered Saline (PBS) (Euroclone, Milan, Italy), minced into 2.5 cm pieces and treated first with 0.2% trypsin and then digested with 0.75 mg/mL Type I-S collagenase (Sigma-Aldrich, Milan, Italy) to remove epithelial cells (Moore et al. 2003). The mixture was then filtered through a 70 μm mesh filter (BD Biosciences, Franklin Lakes, NJ, USA) to separate the dispersed amnion cells from the tissue pieces. Cells were centrifuged at 350 × g for 10 min and resuspended in 15 mL standard growth medium containing Dulbecco’s Modified Eagle’s Medium High Glucose (DMEM-HG) supplemented with 10% fetal calf serum (FCS) (PAA, Pasching, Austria), 1% penicillin/streptomycin (P/S) (Euroclone), 2 mM glutamine (Euroclone), then seeded into 10 cm diameter culture dishes (Corning Life Sciences, Lowell, MA, USA). To calculate the cell growth rate, cells were detached by using 0.2% trypsin and resuspended in PBS, then diluted 1:2 in Trypan Blue (Sigma-Aldrich) and counted with a hemocytometer at different time points (at day 1, 4, 6, 8, 10, 15 and 20) using an inverted microscope. Mean population doubling was obtained according to the formula: PD = (lg Nt−lg N0)/lg 2 (Horisberger 2006), where N0 is the initial cell number and Nt is the cell harvest number at each time point.
For cryopreservation the cells between passages 2 and 8 were suspended in 10% DMEM-HG, 80% FBS, and 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich). Aliquots were dispensed in cryovials at 1–3 × 106 cells per milliliter and slowly frozen (−1 °C/min) to −80 °C in an appropriated container (Nalgene Nunc International, Rochester, NY, USA). After 24 h criovials were transferred to liquid nitrogen.
Stem cell marker expression
The expression of specific mesenchymal stromal cell markers was investigated with retrotranscritpion quantitative PCR (RT-qPCR). RNA was isolated at the 4th passage using the RNeasy kit (Qiagen, Germantown, MD, USA). Primer pairs were designed based on equine gene specific sequence for GAPDH, Oct-4, Sox-2, Nanog, CD34 and CD105. Primer sequences for the characterization protocol of equine stem cells are covered by patent application filed by Fondazione Parco Tecnologico Padano.
Total RNA (1 μg) was treated with DNAse (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocol, to avoid false positive RT-qPCR results due to genomic DNA. RNA was then converted to cDNA using RevertAid H Minus M-MLV RT reverse transcriptase (Fermentas, Burlington, Ontario, Canada). All RT-qPCR reactions were carried out as previously described (Violini et al. 2009). Dissociation curve analysis was run to ensure the absence of non specific PCR products. Samples that did not include reverse transcriptase were included as negative control to monitor DNA contamination and four blank samples were added as qPCR negative controls. RT-qPCR data were analysed using qBasePLUS software (Biogazelle, Ghent, Belgium). GAPDH gene was used as reference for data normalization. PCR efficiency E was calculated from the slope of a serial dilution of representative template (standard curve) and inter-run calibration was used to detect and correct inter-run variation. “Target specific amplification efficiency” and “scale to minimum” parameters were used to obtain a calibrated normalized relative quantity of expression (Hellemans et al. 2007) for the sample analysed.
Amnion cell differentiation
The capacity of the equine amnion cells to differentiate into adipocytes, osteocytes and chondrocytes precursors was also analyzed. Adipogenesis was obtained by culturing cells in 1X StemPro® Differentiation Medium (Invitrogen), at 37 °C for 10 days, whereas untreated cells were grown in standard medium. The adipogenic differentiation was assessed by staining cell monolayers with 2% Oil Red-O (ORO) in isopropanol as described by Vidal et al. (2007). Briefly, cells were fixed with 3.7% formalin for 10 min and permeabilized with 60% isopropanol for 30 s. ORO stain was applied for 10 min and then cells were rinsed with 60% isopropanol for 5 s. Finally, cells were rinsed with distilled water and nuclei counterstained using Mayer’s haematoxylin solution for 10 min. To induce osteogenesis amnion cells were plated in a 6-well-plate at a density of 1 × 104 cells/well and incubated with StemPro Osteogenesis Differentiation kit (Invitrogen®) for a week. To detect the presence of calcium deposits Alizarin Red S (Sigma-Aldrich) staining was carried out as indicated by the manufacturer’s protocol. Briefly cells were fixed with formaldehyde 4% for 30 min and then washed with PBS. Alizarins Red S solution was used at 2% concentration. All experiments were performed in triplicate. The chondrogenic differentiation was performed by incubating for 10 days 1 × 104 cells/well amnion stromal cells in a 6-well-plate with DMEM-HG (PAA), 10% FCS (Euroclone) 1% P/S, 100 nM dexamethasone, 50 μg/mL ascorbic acid 2-phosphate, TGF-β1 (Peprotech, Rock Hill, NJ) and 1X ITS (Insulin, Transferrin and Selenium acid). Amnion cells maintained in regular growth medium without differentiation treatment were stained and used as negative control. To detect mucopolysaccaride deposits in chondrocytes precursors cell pellets were fixed in 10% formalin and embedded in paraffin for Alcian Blue staining and evaluated with light microscopy (Vidal et al. 2007). All chemicals were purchased from Sigma-Aldrich unless otherwise specified.
Results
Cell characteristics
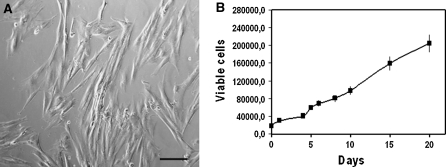
By day 3 of culture in standard growth medium, a clone of adherent mononuclear cells was evident and proliferated uniformly, maintaining homogeneous elongated fibroblast-like morphology (Fig. 1a). These cells were characterized by large nuclei and a spindle shaped appearance. No further changes in cell morphology was observed all through day 20. Amnion cells presented a mean doubling time of 1.78 days (Fig. 1b).
Fig. 1.
Amnion cell line in culture. Adherent cells isolated from amnion presented a uniform fibroblast like morphology; bar = 7 μm (a). The cellular growth rate showed that proliferation capabilities were maintained over 20 days in culture (b). Errors bars represent standard deviation
RT-qPCR analysis
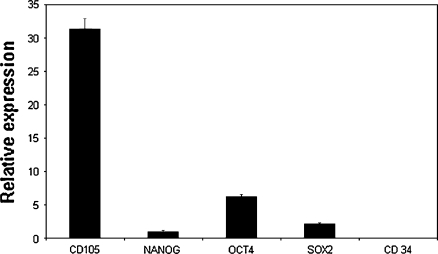
To characterize amnion cell gene expression, RNA was isolated from in vitro cultures between passages 3 and 6 and analyzed by RT-qPCR. Equine amnion cells expressed the mesenchymal stromal cell markers CD105 and Oct-4, known to be expressed also in equine bone marrow mesenchymal stem cells (Violini et al. 2009). The same markers are expressed in human amniotic stromal cells (Insausti et al. 2010) (Fig. 2); moreover the cell line expressed the transcription factors Nanog and Sox-2, involved in maintaining the pluripotency and the cellular undifferentiated state of embryonic stem cells (Marcus et al. 2008; Chambers et al. 2003). The amnion-derived stromal cell line did not express the haematopoietic stem cell marker CD34.
Fig. 2.
RT-qPCR analysis. Plots of the RT-qPCR expression data (y-axis) of mesenchymal stromal marker CD105, Nanog, Oct-4, Sox-2 and CD34 for horse amnion cells. Standard deviations were indicated by vertical bars
Differentiation
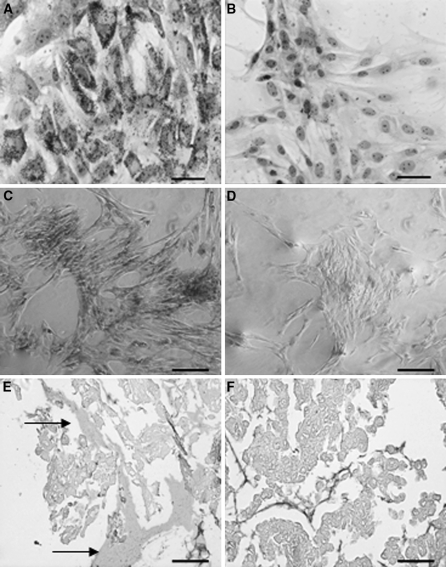
Following adipogenic induction, cells showed in the cytoplasm several accumulated red fat droplets which were detected by ORO staining (Fig. 3a). In comparison untreated cells stained for lipid droplets did not show these fat bodies, indicating the occurrence of the differentiation process (Fig. 3b). Amnion cells grown in osteoblast differentiation medium showed deep orange-red staining of calcium deposits clearly visible as Alizarin Red S–calcium complexes (Fig. 3c). The untreated negative control is shown in Fig. 3d.
Fig. 3.
Differentiation of amnion-derived stromal cells. Cells cultured in adipogenic induction media for 10 days showed accumulated red fat droplets by ORO staining only in induced amnion cells cytoplasm (a), not present in untreated control cells grown in regular medium (b) (bar = 10 μm). Nuclei were counterstained with hematoxylin. Alizarin Red S staining detected calcium deposits upon osteogenic induction (c) versus untreated negative control (d) (bar = 25 μm). Pellets cultured from MSCs induced into chondrocyte precursors showed positive extracellular matrix staining by Alcian Blue (e) (arrows) compared to the untreated negative control (f). Cells were counterstained with Safranin (bar = 200 μm). (color figure online)
Upon chondrogenic induction the cell line extracellular matrix was clearly identified after Alcian Blue staining (Fig. 3e). Untreated control showed no matrix staining (Fig. 3f).These studies demonstrated the potential of the equine amnion cell line to differentiate into adipocyte, osteoblast and chondrocyte precursors in culture.
Discussion
Recently mesenchymal stromal cells have been deeply investigated as important source of undifferentiated stem cells for human medicine and veterinary applications (Sensebé and Bourin 2009). As they can potentially differentiate into different specific cell types, they have become promising candidates in tissue engineering, wound healing and tissue repair. So far horse stem cells have been isolated from a variety of tissues such as bone marrow, fat and umbilical cord. Still alternative sources of cells able to differentiate in mesoderm lineage are necessary.
The present study showed the isolation and characterization of a stromal cell line from explanted horse amniotic membrane. Under standard culture conditions, equine amnion stromal adherent cells showed uniformly elongated morphology. Regarding the protein identification, the use of immunocytochemistry for antigen detection in horse samples is currently limited due to lack of specific monoclonal antibodies commercially available. Therefore to investigate the expression of proteins of interest the RT-qPCR approach was used, as previously reported (Konnai et al. 2003; Badie-Mahdavi et al. 2005).
Cells were shown to express the mesenchymal stromal cells marker CD105 and the pluripotent stem cell marker Oct-4; interestingly cells also expressed Nanog and Sox-2 genes, known to be involved in self-renewal and pluripotency of both inner cell mass (ICM) and embryonic stem (ES) cells (Guo et al. 2009).
The equine amnion-derived cell line express high levels of CD105, confirming its stromal origin. Similar CD105 expression data were reported by Moon et al. (2008) and Stadler et al. (2008) for human amnion-derived mesenchymal stem cells (MSCs).
As reported in previous studies on human stem cells (Ulloa-Montoya et al. 2007; Yalvac et al. 2010), Oct-4, Nanog and Sox-2 showed different relative gene expression in the cell line isolated and this is probably due to different regulatory mechanisms of these genes (Barrand and Collas 2010). Amnion cells did not express CD34, a known cell marker expressed by hematopoietic stem cells.
Cells showed the potential for in vitro differentiation into adipocytes as demonstrated by the presence of red granules identified as lipid droplets in the cytoplasm, similar to those already observed in horse adipose tissue-derived stem cell (Mambelli et al. 2009) and stem cells differentiated from umbilical cord blood (Koch et al. 2007). The amnion-derived stromal cells also had the potential to be differentiated into osteoblasts clearly demonstrated by calcium deposit accumulation. Upon chondrogenesis induction Alcian blue staining revealed a homogeneous deposition of proteoglycan in amnion cells. The expression analysis and the differentiation processes obtained clearly confirmed the potential for mesodermal lineage differentiation and the mesenchymal stromal characteristics of the amnion cell line.
This study showed that horse amnion represents a valuable source of stromal cells alternative to bone marrow and fat stem cells. Like umbilical cord amnion tissue is available at any foal birth without any invasive procedure. From amnion tissue both epithelial and stromal amnion cells can be obtained, representing an interesting cell types to be tested in horse for differentiation processes other than the mesenchymal lineage. Recently placenta-derived stem cells have attracted attention as a novel cell source for cell transplantation (Gucciardo et al. 2009) as, among other features; the harvest of such cells does not require any invasive treatment. Interestingly, being fetal cells, amnion-derived cells could offer a number of therapeutic advantages over adult stem cells as they can differentiate in vitro into diverse cells types, have low immunogenicity and anti-inflammatory function, making them well suited for cell replacement therapy (Parolini et al. 2008). The protocol standardization for isolation and maintenance of equine amnion-derived mesenchymal stromal cells (AMSCs) in culture presented here is a significant step to facilitate the creation of an equine cell bank for application in veterinary medicine.
Acknowledgments
The authors thank Dr. John Williams for the critical reading of the manuscript.
Ethical standard The care and use of experimental animals complied with local and national animal welfare laws, guidelines and policies.
Contributor Information
Stefania Violini, Phone: +39-0371-4662327, FAX: +39-0371-4662349.
Paola Mariani, Email: paola.mariani@tecnoparco.org.
References
- Badie-Mahdavi H, Lu X, Behrens MM, Bartfai T. Role of galanin receptor 1 and galanin receptor 2 activation in synaptic plasticity associated with 3′,5′-cyclic AMP response element-binding protein phosphorylation in the dentate gyrus: studies with a galanin receptor 2 agonist and galanin receptor 1 knockout mice. Neuroscience. 2005;133:591–604. doi: 10.1016/j.neuroscience.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Barrand S, Collas P. Chromatin states of core pluripotency-associated genes in pluripotent, multipotent and differentiated cells. Biochem Biophys Res Commun. 2010;391:762–767. doi: 10.1016/j.bbrc.2009.11.134. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/S0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Fortier LA, Nixon AJ, Williams J, Cable CS. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res. 1998;59:1182–1187. [PubMed] [Google Scholar]
- Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007;25:3143–3154. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- Gucciardo L, Lories R, Ochsenbein-Kölble N, Done’ E, Zwijsen A, Deprest J. Fetal mesenchymal stem cells: isolation, properties and potential use in perinatology and regenerative medicine. BJOG. 2009;116:166–172. doi: 10.1111/j.1471-0528.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- Guo J, Li ZC, Feng YH. Expression and activation of the reprogramming transcription factors. Biochem Biophys Res Commun. 2009;390:1081–1086. doi: 10.1016/j.bbrc.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger MA. A method for prolonged survival of primary cell lines. In Vitro Cell Dev Biol Anim. 2006;42:143–148. doi: 10.1290/0511081.1. [DOI] [PubMed] [Google Scholar]
- Ilancheran S, Moodley Y, Manuelpillai U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta. 2009;30:2–10. doi: 10.1016/j.placenta.2008.09.009. [DOI] [PubMed] [Google Scholar]
- In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- Insausti CL, Blanquer M, Bleda P, Iniesta P, Majado MJ, Castellanos G, Moraleda JM. The amniotic membrane as a source of stem cells. Histol Histopathol. 2010;25:91–98. doi: 10.14670/HH-25.91. [DOI] [PubMed] [Google Scholar]
- Kim J, Kang HM, Kim H, Kim MR, Kwon HC, Gye MC, Kang SG, Yang HS, You J. Ex vivo characteristics of human amniotic membrane-derived stem cells. Cloning Stem Cells. 2007;9:581–594. doi: 10.1089/clo.2007.0027. [DOI] [PubMed] [Google Scholar]
- Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007;30:7–26. doi: 10.1186/1472-6750-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnai S, Usui T, Ohashi K, Onuma M. The rapid quantitative analysis of bovine cytokine genes by real-time RT-PCR. Vet Microbiol. 2003;94:283–294. doi: 10.1016/S0378-1135(03)00119-6. [DOI] [PubMed] [Google Scholar]
- Mambelli LI, Santos EJ, Frazão PJ, Chaparro MB, Kerkis A, Zoppa AL, Kerkis I. Characterization of equine adipose tissue-derived progenitor cells before and after cryopreservation. Tissue Eng Part C Methods. 2009;15:87–94. doi: 10.1089/ten.tec.2008.0186. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Coyne TM, Rauch J, Woodbury D, Black IB. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76:130–144. doi: 10.1111/j.1432-0436.2007.00194.x. [DOI] [PubMed] [Google Scholar]
- Moon JH, Lee JR, Jee BC, Suh CS, Kim SH, Lim HJ, Kim HK. Successful vitrification of human amnion-derived mesenchymal stem cells. Hum Reprod. 2008;238:1760–1770. doi: 10.1093/humrep/den202. [DOI] [PubMed] [Google Scholar]
- Moore RM, Silver RJ, Moore JJ. Physiological apoptotic agents have different effects upon human amnion epithelial and mesenchymal cells. Placenta. 2003;24:173–180. doi: 10.1053/plac.2002.0886. [DOI] [PubMed] [Google Scholar]
- Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring H-J, Evangelista M, Hennerbichler S, Liu B, Magatti R, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido TC, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Reidler H, Skuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- Reed SA, Johnson SE. Equine umbilical cord blood contains a population of stem cells that express Oct4 and differentiate into mesodermal and endodermal cell types. J Cell Physiol. 2008;215:329–336. doi: 10.1002/jcp.21312. [DOI] [PubMed] [Google Scholar]
- Sensebé L, Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87:S49–S53. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- Stadler G, Hennerbichler S, Lindenmair A, Peterbauer A, Hofer K, Griensven M, Gabriel C, Redl H, Wolbank S. Phenotypic shift of human amniotic epithelial cells in culture is associated with reduced osteogenic differentiation in vitro. Cytotherapy. 2008;107:743–752. doi: 10.1080/14653240802345804. [DOI] [PubMed] [Google Scholar]
- Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105:215–228. doi: 10.1254/jphs.CR0070034. [DOI] [PubMed] [Google Scholar]
- Ulloa-Montoya F, Kidder BL, Pauwelyn KA, Chase LG, Luttun A, Crabbe A, Geraerts M, Sharov AA, Ko MSH, Piao Y, Hu W-S, Verfaillie CM. Comparative transcriptome analysis of embryonic and adult stem cells with extended and limited differentiation capacity. Genome Biol. 2007;8:R163. doi: 10.1186/gb-2007-8-8-r163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal MA, Kilroy GE, Lopez MJ, Johnson JR, Moore RM, Gimble JM. Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg. 2007;36:613–622. doi: 10.1111/j.1532-950X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- Violini S, Ramelli P, Pisani LF, Gorni C, Mariani P. Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol. 2009;10:29. doi: 10.1186/1471-2121-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalvac ME, Ramazanoglu M, Rizvanov AA, Sahin F, Bayrak OF, Salli U, Palotas A, Kose GT. Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: implications in neo-vascularization, osteo-, adipo- and neurogenesis. Pharmacogenomics J. 2010;10(2):105–113. doi: 10.1038/tpj.2009.40. [DOI] [PubMed] [Google Scholar]