Abstract
AIM: To investigate the association between single nucleotide polymorphisms (SNPs) in intercellular adhesion molecule-1 (ICAM-1) and the risk, biological behavior and prognosis of gastric cancer (GC) in Chinese population.
METHODS: The study group consisted of 332 GC patients and 380 healthy controls. Genotyping was performed using polymerase chain reaction and the results were confirmed by sequencing. The association of ICAM-1 K469E polymorphisms and the risk of GC were studied, and the correlation of ICAM-1 K469E polymorphisms with the clinicopathological parameters and prognosis of the patients with complete clinical and follow-up data was analyzed.
RESULTS: Carriers of AA genotype had a significantly increased risk of GC compared with carriers of AG and GG genotypes [odds ratios: 1.36; 95% confidence interval (CI): 1.01-1.84; P = 0.041]. GC patients with AA genotype were more prone to distant metastasis than those carrying AG and GG genotypes (18.9% vs 7.0%, respectively; P = 0.002). In addition, patients at stage IV had significantly more carriers of AA genotype than those of AG and GG genotype (27.4% vs 16.9%, respectively; P = 0.046). Follow-up study showed that the overall cumulative survival rate was 23.7% in AA genotype group and 42.9% in AG and GG genotypes group. In univariate analysis, AA genotype was correlated with the overall cumulative survival (P = 0.034). But in multivariate analysis, ICAM-1 polymorphism was not an independent prognostic factor for the overall survival (relative risk, 1.145; 95% CI: 0.851-1.540; P = 0.370).
CONCLUSION: Polymorphisms of ICAM-1 K469E can be a useful biomarker for identifying individuals with higher risk of GC, predicting disease progression, and guiding individualized treatment.
Keywords: Intercellular adhesion molecule-1, Gene polymorphism, Gastric cancer, Risk, Prognosis
INTRODUCTION
Gastric cancer (GC) is the fourth most common cancer in the world and has the second highest mortality rate[1]. GC is a biologically and genetically heterogenous tumor and develops as a result of the interplay among genetic and environmental factors. Genetic polymorphism plays a role in determining how hosts respond to various environmental factors[2-4].
As a risk factor for GC, Helicobacter pylori (H. pylori) infection is closely correlated with the occurrence of GC. A number of studies have confirmed that many polymorphisms of inflammation-related genes are significantly associated with the risk of GC[5-8]. Intercellular adhesion molecule-1 (ICAM-1) is a cell adhesion molecule belonging to the immunoglobulin superfamily and is the ligand of β2 integrin (LFA-1 and Mac-1)[9]. H. pylori infection can induce increased expression of ICAM-1, possibly due to the stimulation of local mucosal inflammation and inflammatory cytokines. ICAM-1 is expressed in various cell types, including leukocytes, epithelial and endothelial cells, fibroblasts and keratinocytes[9,10]. ICAM-1 plays an important role in cell-to-cell, cell-to-extracellular matrix interaction and cell signaling. Two single-nucleotide polymorphisms (SNPs) in the ICAM1 gene have been recognized, the first at position +241 (+241G/A or G241R) located in exon 4 (GGG→AGG; Gly→Arg), and the second one at position +469 (+469 A/G or K469E) located in exon 6 and coding the Ig-like domain 5 (AAG→GAG; Lys→Glu)[11]. Some studies have shown that ICAM-1 plays an important role in the development and progression of human cancers. Overexpression of ICAM-1 in melanoma and renal cell carcinoma has been reported[12,13]. There is also a slightly increased expression of ICAM-1 in other tumors, such as gastric, breast, and colorectal cancers[14-16]. The soluble form of ICAM-1, sICAM-1, is partially detectable in the serum of healthy subjects, but its level is elevated in malignancies. It has been observed that sICAM-1 serum level was positively correlated with tumor size, lymph node and distant metastasis of cancers, such as breast cancer, pancreatic cancer, gastric cancer, lung cancer and hepatocellular carcinoma[17-22]. The SNP in ICAM1 gene was also found associated with the risk of cancers, including breast cancer, colorectal cancer and melanoma[23-28].
There has been no report on the relationship between ICAM1 gene polymorphisms and GC risk. The polymorphism of G241R is rare in Chinese populations[28,29]. In this study, we aimed to examine the frequency of the ICAM-1 K469E polymorphism in the Chinese population and its correlation with the risk of GC using a large case-control cohort from our hospital. We also analyzed the relationship between K469E polymorphism and the clinicopathological parameters and prognosis of GC patients with complete clinical and follow-up data.
MATERIALS AND METHODS
Patients and specimens
In this hospital-based case-control study, histologically normal tissue specimens of 332 patients (124 females and 208 males, median age 59 years, range 29-91 years) who underwent radical gastrectomy for GC without any adjuvant therapy prior to surgery in the Beijing Cancer Hospital from May 1999 to June 2004 were collected prospectively. Various clinicopathological data were evaluated or collected from patient files by a pathologist. Cancer staging followed the 7th edition of the TNM classification of the International Union Against Cancer (UICC)[30]. Of the 332 patients, 301 patients had complete follow-up records. The survival time was counted in months from the time of surgery to the date of death.
A total of 380 subjects (186 females and 194 males, median age 57 years, range 25-84 years) who received endoscopy in the Beijing Cancer Hospital from 2002 to 2005 with a pathological diagnosis of superficial gastritis served as controls. All patients and controls were of Chinese Han descent and lived in and around Beijing.
A total of 102 serum samples were provided by the Beijing Cancer Hospital tissue bank. H. pylori infection was detected by immunohistochemistry.
Experimental methods
Histologically normal tissues from patients and biopsied tissues from controls, fixed in formalin and embedded in paraffin, were used for extraction of genomic DNA. Tissues were dewaxed using xylene and ethanol and then dried. After addition of 1 mL lysis buffer and 10 μL proteinase K solution (20 mg/mL), the tissues were incubated at 37 °C overnight. The solution was extracted with equal volumes of saturated phenol, phenol : chloroform (1:1), and chloroform : isoamyl alcohol (24:1). After addition to the supernatant of 1/10 volume of NaOAc (3 mol/L) and 2.5 times the volume of ice-cold ethanol, the solution was precipitated at -20 °C overnight. The precipitate was washed with 70% ethanol to remove the salt. After the precipitate was dissolved in 1 × TE solution (pH 7.6), the DNA was stored at -20 °C.
According to GenBank full-length sequence of human ICAM1 gene, we applied Primer 5.0 software to design 469K/E locus-specific primers. The primers used were 5’-GCTCAAGTGTCTAAAGGATGGC-3’ (forward) and 5’-CTCACAGAGCACATTCACGG-3’ (reverse). The annealing temperature was 60 °C and the length of polymerase chain reaction (PCR) products was 148 bp. The PCR products were sequenced using an ABI377 automatic sequencer (Applied Biosystems, Foster City, CA, United States).
sICAM-1 was measured using an enzyme linked immunosorbent assay (ELISA) kit. ICAM-1 expression in GC tissues was detected by immunohistochemistry using an ICAM-1 mouse monoclonal antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, United States). As a membrane protein, the positive staining for ICAM-1 was located in the cytoplasm and at the cell membrane. If > 10% cells were positively stained in each slice, the tissue was considered ICAM-1 positive[14]. H. pylori infection was detected by immunohistochemistry using an H. pylori rabbit monoclonal antibody (1:200; Zeta Corporation, Sierra Madre, CA, United States).
Statistical analysis
All analyses were performed using SPSS statistical analysis software, version 10.0 (SPSS, Chicago, IL, United States). Differences in age, sex, H. pylori infection, tobacco smoking, alcohol consumption, and the distribution of the genetic polymorphism between cases and controls were calculated using Pearson’s χ2 test. It was also determined if the genotype distribution was consistent with Hardy-Weinberg equilibrium. P < 0.05 (both sides) was considered statistically significant.
The correlation between the polymorphism and clinicopathologic parameters of GC was also examined by Pearson’s χ2 test, and P < 0.05 (both sides) was considered statistically significant. The Kaplan-Meier method and log-rank test were used to evaluate the correlation between the polymorphism and patient survival. Odds ratios (OR) and confidence intervals (CI) (95%) were calculated by Cox regression model to examine the impact of the polymorphism on prognosis. P value < 0.05 was considered statistically significant.
RESULTS
Characteristics of GC patients and controls
Baseline characteristics of the 332 GC patients and 380 control subjects are summarized in Table 1. There were more males among the patients with GC (62.7%) compared with the control group (51.1%, P = 0.002). However, no significant difference was observed between the cases and controls in age, H. pylori infection, tobacco smoking, and alcohol consumption (Figure 1).
Table 1.
General status of gastric cancer patients and controls
| Variables | Patients (n = 332) (%) | Controls (n = 380) (%) | P value |
| Age (yr) | |||
| mean ± SE | 57.37 ± 0.68 | 56.92 ± 0.66 | 0.638 |
| Gender | |||
| Male | 208 (62.7) | 194 (51.1) | 0.002 |
| Female | 124 (37.3) | 185 (48.9) | |
| Helicobacter pylori infection | |||
| Yes | 138 (41.6) | 156 (41.1) | 0.89 |
| No | 194 (58.4) | 224 (58.9) | |
| Tobacco smoking | |||
| Yes | 86 (25.9) | 82 (21.6) | 0.175 |
| No | 246 (74.1) | 298 (78.4) | |
| Alcohol consumption | |||
| Yes | 63 (19.0) | 73 (19.2) | 0.937 |
| No | 269 (81.0) | 307 (80.8) |
Figure 1.
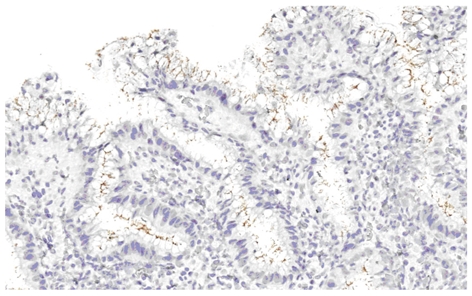
Helicobacter pylori infection was detected by immunohistochemistry.
ICAM-1 +469 A/G polymorphism and its relationship with GC risk
As is shown in Table 2, the frequency of the AA genotype was 57.2% and 49.2% in patients and controls, whereas the frequency of the AG genotype was 34.9% and 42.4% in patients and controls, respectively. On the other hand, the frequency of GG genotype was 7.8% and 8.4% in patients vs controls, respectively. The most common genotype was AA followed by AG. Significantly more AA carriers were found among the patients with GC compared with the controls (P = 0.032, Figure 2). It is noticeable that both patients and controls were in Hardy-Weinberg equilibrium.
Table 2.
Genotype and allele distribution of ICAM-1 +469 A/G polymorphism
| Patients(n = 332) (%) | Controls(n = 380) (%) | P valuea | |
| Genotype | |||
| AA | 190 (57.2) | 187 (49.2) | 0.032b |
| AG | 116 (34.9) | 169 (42.4) | |
| GG | 26 (7.8) | 24 (8.4) | |
| Allele | |||
| A | 496 (74.7) | 543 (71.4) | 0.070 |
| G | 168 (25.3) | 217 (28.6) |
a: Pearson χ2 test; b: AA vs AG + GG. ICAM-1: Intercellular adhesion molecule-1.
Figure 2.
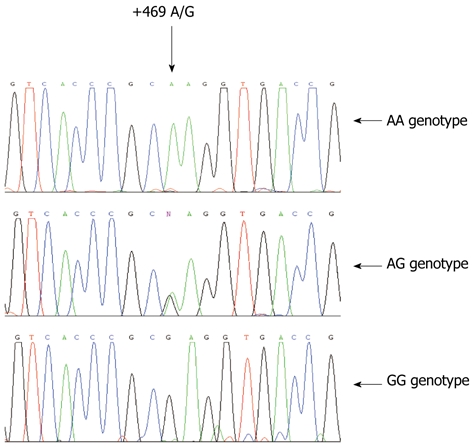
Sequence data of intercellular adhesion molecule-1 K469E genotype. These three graphs represent AA, AG and GG genotypes.
Considering the low frequency of the GG genotype, we combined the GG and AG genotypes for use as the reference. After adjustment for age, sex, H. pylori infection, smoking, and alcohol consumption, subjects with AA genotype had a 1.36-fold increase in GC risk compared with those with AG + GG genotypes (OR = 1.36, 95% CI = 1.01-1.84, P = 0.041).
Association between ICAM-1 +469 A/G polymorphism and clinicopathological parameters of GC
The potential associations of the ICAM-1 +469 A/G polymorphism with tumor characteristics are presented in Table 3. Among the 332 patients with GC, the frequencies of the three genotypes were 57.2% (AA, 190/332), 34.9% (AG, 116/332), and 7.8% (GG, 26/332), respectively. No correlation was found between +469 A/G genotypes and tumor size, differentiation, presence of lymph node metastases and vascular invasion, or age and gender at diagnosis. However, AA genotype carriers had distant metastasis more frequently than AG and GG carriers (18.9% vs 7.0%, P = 0.002). In addition, there were more stage IV patients carrying the AA genotype compared with patients with the AG and GG genotypes (27.4% vs 16.9%, P = 0.046).
Table 3.
Association between ICAM-1 +469 A/G polymorphism and clinicopathological parameters of patients with gastric cancer
| Variables | Total |
ICAM-1 +469 A/G genotype |
P value | |
| (n = 332) | AA(n = 190) (%) | AG + GG(n = 142) (%) | ||
| Age (yr) | 0.733 | |||
| mean ± SE | 57.17 ± 0.94 | 57.64 ± 0.99 | ||
| Gender | 0.355 | |||
| Male | 208 | 115 (60.5) | 93 (65.5) | |
| Female | 124 | 75 (39.5) | 49 (34.5) | |
| Tumor size | 0.154 | |||
| ≤ 5cm | 174 | 106 (55.8) | 68 (47.9) | |
| > 5cm | 158 | 84 (44.2) | 74 (52.1) | |
| Differentiation | 0.760 | |||
| Well | 120 | 70 (36.8) | 50 (35.2) | |
| Poor | 212 | 120 (63.2) | 92 (34.8) | |
| Lymph node metastasis | 0.928 | |||
| Negative | 102 | 58 (30.5) | 44 (31.0) | |
| Positive | 230 | 132 (69.5) | 98 (69.0) | |
| Vascular invasion | 0.408 | |||
| Negative | 176 | 97 (51.1) | 79 (55.6) | |
| Positive | 156 | 93 (48.9) | 63 (44.4) | |
| Distant metastasis | 0.002 | |||
| Negative | 286 | 154 (81.1) | 132 (93.0) | |
| Positive | 46 | 36 (18.9) | 10 (7.0) | |
| TNM stages | 0.046a | |||
| I | 62 | 35 (18.4) | 27 (19.0) | |
| II | 60 | 35 (18.4) | 25 (17.6) | |
| III | 134 | 68 (35.8) | 66 (46.5) | |
| IV | 76 | 52 (27.4) | 24 (16.9) | |
a: IV vs I + II + III. ICAM-1: Intercellular adhesion molecule-1.
ICAM-1 +469 A/G polymorphism and GC prognosis
To evaluate whether the ICAM-1 +469 A/G polymorphism in GC correlates with a worse prognosis, Kaplan-Meier survival curves were constructed using overall cumulative survival to compare the patients with AA genotype to those with AG and GG genotypes. Our data revealed that AA genotype was correlated with overall cumulative survival (P = 0.034, log-rank test; Figure 3). The overall cumulative survival rate was 23.7% in AA genotype group and 42.9% in AG and GG genotypes group. In addition, we performed a multivariate analysis including ICAM-1 polymorphism, age, sex, tumor size, differentiation, depth of invasion, lymph node metastasis, distant metastasis, and TNM stage for GC, and found that ICAM-1 polymorphism is not an independent prognostic factor for the overall survival (relative risk, 1.145; 95% CI, 0.851-1.540; P = 0.370).
Figure 3.
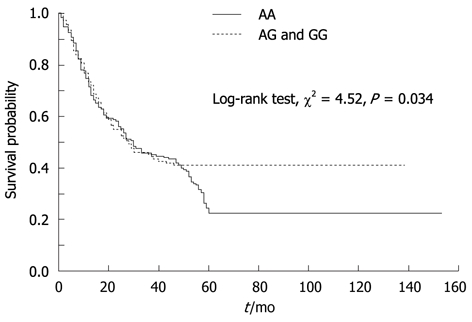
Kaplan-Meier survival analyses of gastric cancer patients.
ICAM-1 +469 A/G polymorphism and sICAM-1 and ICAM-1 expression in GC
Among the 332 patients with GC, 102 had available serum samples. The serum sICAM-1 levels in these patients were measured using an ELISA kit. ICAM-1 immunohistochemical staining of paraffin sections from these patients showed that ICAM-1 was mainly expressed in the invasive front cells (Figure 4). Pearson’s χ2 test showed that the ICAM-1 +469 A/G polymorphism was not significantly associated with sICAM-1 or ICAM-1 expression in GC (Table 4).
Figure 4.
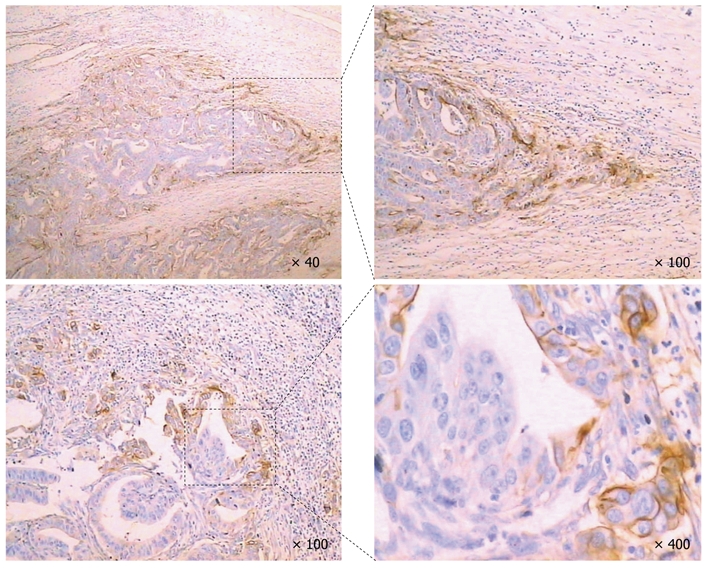
Intercellular adhesion molecule-1 expression in gastric cancer. Intercellular adhesion molecule-1 (ICAM-1) was mainly expressed in the invasive front cells.
Table 4.
ICAM-1 +469 A/G polymorphism and sICAM-1 and ICAM-1 expression in gastric cancer
| Characteristics | Total |
Genotype |
P value | |
| (n = 102) | AA | AG + GG | ||
| Serum sICAM-1 | 0.990 | |||
| mean ± SE | 176.00 ± 7.72 | 175.85 ± 9.40 | ||
| ICAM-1 expression | 0.197 | |||
| Positive | 29 | 13 (44.8) | 16 (55.2) | |
| Negative | 73 | 43 (58.9) | 30 (41.1) | |
ICAM-1: Intercellular adhesion molecule-1; sICAM-1: The soluble form of ICAM-1.
DISCUSSION
In the present case-control study, we found that ICAM-1 K469E polymorphisms were associated with significantly increased risk of GC. Consistent with our results, Theodoropoulos et al[31] reported that this SNP in ICAM1 gene was associated with increased risk of colorectal carcinoma. Gastrointestinal carcinomas might largely be secondary to inflammation and immune response. Gastric carcinogenesis is closely related to chronic inflammatory lesion in gastric mucosa mainly caused by H. polyri infection. ICAM-1 plays a pivotal role in leucocyte migration across endothelial cells and facilitates leukocyte recruitment into inflammatory sites. K469E amino acid changes in the ICAM1 gene may influence its functional role, as it is located at the Mac-1 binding domain and the immunoglobin-like domain 5. The ICAM-1 K469E polymorphism may result in the functional alteration of the gene product that facilitates the inflammation and immune response. The mixture of cytokine produced in inflammation and immune response contributes to the establishment of oncogenic microenvironment that plays a key role in initiation of gastric carcinogenesis by stimulating angiogenesis, damaging DNA and epithelial cell proliferation and subsequent malignant transformation[32,33]. For example, the pro-inflammatory and acid inhibiting properties of TNFα seem to enhance H. pylori oncogenic or other effects on gastric mucosa. In another study, we found that ICAM-1 K469E polymorphism was strongly associated with the progression of gastric lesions from gastritis to dysplasia (data not shown).
Since ICAM-1 K469E polymorphism could lead to the functional alteration of the gene product, it may influence its role in pathogenesis as well as in the progression and subsequent prognosis. This study revealed that K469E polymorphism was strongly associated with distant metastasis and advanced staging (stage IV) of GC. In addition, this polymorphism was correlated with poor survival of the patients, although there was no correlation between polymorphism and expression and soluble level of ICAM-1. Our results showed that ICAM-1 was mainly expressed in the invasive front cells. These findings are in agreement with previous reports on the involvement of ICAM-1 in tumor progression[34-37]. The possible mechanism of this phenomenon is that tumor cells with high ICAM-1 expression could bind to infiltrating lymphocytes through an ICAM-1/LFA-1 interaction. As a result, cancer cells could become detached from the tumor mass and migrate into the blood circulation. Additionally, cells would not be damaged when transiting through the blood vessels and could easily adhere to the capillaries or lymphatic sinus, leading to metastasis.
Our data analysis relied on extracted genomic DNA from tissue samples preserved in paraffin blocks. Rae et al[38] reported that the fixation process used for making paraffin blocks did not affect genotype detection. They found that the results of genotype detection with genomic DNA from paraffin blocks were consistent with the results of genotype detection using genomic DNA from peripheral blood and suggested that accurate and reliable genotyping results with paraffin samples could be achieved.
To the best of our knowledge, this is the first evidence to indicate that individuals carrying the AA genotype of the ICAM-1 K469E polymorphism might be more susceptible to GC. In addition, patients with the AA genotype were more likely to develop distant metastasis and have a higher risk of death compared with other patients. These results suggested that the ICAM-1 K469E polymorphism could be used as a new molecular marker to screen for individuals with higher risk of GC, predict disease progression, and guide individualized treatment.
COMMENTS
Background
Gastric cancer (GC) is a biologically and genetically heterogenous tumor and develops as a result of the interplay among genetic and environmental factors. Genetic polymorphism plays a role in determining how hosts respond to various environmental factors. A number of studies have confirmed that polymorphisms of many inflammation-related genes are significantly associated with the risk of GC.
Research frontiers
According to the authors of this paper, there has been no report on the relationship between intercellular adhesion molecule-1 (ICAM-1) gene polymorphisms and the risk, biological behavior and prognosis of GC.
Innovations and breakthroughs
In this study, the authors investigated the association between single nucleotide polymorphisms in ICAM-1 and the risk, biological behavior and prognosis of GC in Chinese population. This is the first evidence to indicate that individuals carrying the AA genotype of the ICAM-1 K469E polymorphism might be more susceptible to GC. In addition, patients with AA genotype were more likely to develop distant metastasis and have a higher risk of death compared with other patients.
Applications
The ICAM-1 K469E polymorphism could be used as a new molecular marker to screen for individuals with higher risk of GC, predict disease progression, and guide individualized treatment.
Terminology
ICAM-1 is a cell adhesion molecule belonging to the immunoglobulin superfamily and is the ligand of β2 integrin (LFA-1 and Mac-1). ICAM-1 plays an important role in cell-to-cell, cell-to-extracellular matrix interaction and cell signaling.
Peer review
The authors investigated K469E polymorphism of ICAM-1 in patients with gastric cancer. The number of cases enrolled in this study is enough and the results are very important.
Footnotes
Supported by Grants from Beijing Municipal Science and Technology Commission NOVA Program, No. 2009BG-02; the National High Technology Research and Development Program of China, No. 2006AA02A402; the Major State Basic Research Program of China, No. 2004CB518702
Peer reviewer: Takaaki Arigami, MD, PhD, Department of Surgical Oncology and Digestive Surgery, Field of Oncology, Kagoshima University Graduate School of Medical and Dental Sciences, 8-35-1 Sakuragaoka, Kagoshima 891-0175, Japan; Salvatore Gruttadauria, MD, Assistant Professor, Abdominal Transplant Surgery, ISMETT, Via E. Tricomi, 190127 Palermo, Italy
S- Editor Cheng JX L- Editor Ma JY E- Editor Zhang DN
References
- 1.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 Suppl 8:S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Sugimoto M, Yamaoka Y, Furuta T. Influence of interleukin polymorphisms on development of gastric cancer and peptic ulcer. World J Gastroenterol. 2010;16:1188–1200. doi: 10.3748/wjg.v16.i10.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Lu Y, Ding YB, Ke Q, Hu ZB, Yan ZG, Xue Y, Zhou Y, Hua ZL, Shu YQ, et al. Promoter polymorphisms of IL2, IL4, and risk of gastric cancer in a high-risk Chinese population. Mol Carcinog. 2009;48:626–632. doi: 10.1002/mc.20502. [DOI] [PubMed] [Google Scholar]
- 4.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 5.Sugimoto M, Furuta T, Yamaoka Y. Influence of inflammatory cytokine polymorphisms on eradication rates of Helicobacter pylori. J Gastroenterol Hepatol. 2009;24:1725–1732. doi: 10.1111/j.1440-1746.2009.06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631–636. doi: 10.1093/carcin/bgh349. [DOI] [PubMed] [Google Scholar]
- 7.Togawa S, Joh T, Itoh M, Katsuda N, Ito H, Matsuo K, Tajima K, Hamajima N. Interleukin-2 gene polymorphisms associated with increased risk of gastric atrophy from Helicobacter pylori infection. Helicobacter. 2005;10:172–178. doi: 10.1111/j.1523-5378.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Miao X, Tan W, Ning B, Liu Z, Hong Y, Song W, Guo Y, Zhang X, Shen Y, et al. Identification of functional genetic variants in cyclooxygenase-2 and their association with risk of esophageal cancer. Gastroenterology. 2005;129:565–576. doi: 10.1016/j.gastro.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 10.Braun C, Zahn R, Martin K, Albert E, Folwaczny C. Polymorphisms of the ICAM-1 gene are associated with inflammatory bowel disease, regardless of the p-ANCA status. Clin Immunol. 2001;101:357–360. doi: 10.1006/clim.2001.5118. [DOI] [PubMed] [Google Scholar]
- 11.Vora DK, Rosenbloom CL, Beaudet AL, Cottingham RW. Polymorphisms and linkage analysis for ICAM-1 and the selectin gene cluster. Genomics. 1994;21:473–477. doi: 10.1006/geno.1994.1303. [DOI] [PubMed] [Google Scholar]
- 12.Natali P, Nicotra MR, Cavaliere R, Bigotti A, Romano G, Temponi M, Ferrone S. Differential expression of intercellular adhesion molecule 1 in primary and metastatic melanoma lesions. Cancer Res. 1990;50:1271–1278. [PubMed] [Google Scholar]
- 13.Tomita Y, Nishiyama T, Watanabe H, Fujiwara M, Sato S. Expression of intercellular adhesion molecule-1 (ICAM-1) on renal-cell cancer: possible significance in host immune responses. Int J Cancer. 1990;46:1001–1006. doi: 10.1002/ijc.2910460609. [DOI] [PubMed] [Google Scholar]
- 14.Maruo Y, Gochi A, Kaihara A, Shimamura H, Yamada T, Tanaka N, Orita K. ICAM-1 expression and the soluble ICAM-1 level for evaluating the metastatic potential of gastric cancer. Int J Cancer. 2002;100:486–490. doi: 10.1002/ijc.10514. [DOI] [PubMed] [Google Scholar]
- 15.Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, Denissenko MF. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis. 2005;26:943–950. doi: 10.1093/carcin/bgi070. [DOI] [PubMed] [Google Scholar]
- 16.Ksiazek K, Mikuła-Pietrasik J, Catar R, Dworacki G, Winckiewicz M, Frydrychowicz M, Dragun D, Staniszewski R, Jörres A, Witowski J. Oxidative stress-dependent increase in ICAM-1 expression promotes adhesion of colorectal and pancreatic cancers to the senescent peritoneal mesothelium. Int J Cancer. 2010;127:293–303. doi: 10.1002/ijc.25036. [DOI] [PubMed] [Google Scholar]
- 17.Eggeman H, Stöblen F, Thill M, Korlach S, Schmid P, Lüftner D, Elling D, Taran FA, Kümmel S, Landt S. Influence of a dose-dense adjuvant chemotherapy on sVCAM-1/sICAM-1 serum levels in breast cancer patients with 1-3 positive lymph nodes. Anticancer Res. 2011;31:2617–2622. [PubMed] [Google Scholar]
- 18.Brand RE, Nolen BM, Zeh HJ, Allen PJ, Eloubeidi MA, Goldberg M, Elton E, Arnoletti JP, Christein JD, Vickers SM, et al. Serum biomarker panels for the detection of pancreatic cancer. Clin Cancer Res. 2011;17:805–816. doi: 10.1158/1078-0432.CCR-10-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakata B, Hori T, Sunami T, Ogawa Y, Yashiro M, Maeda K, Sawada T, Kato Y, Ishikawa T, Hirakawa K. Clinical significance of serum soluble intercellular adhesion molecule 1 in gastric cancer. Clin Cancer Res. 2000;6:1175–1179. [PubMed] [Google Scholar]
- 20.Alexiou D, Karayiannakis AJ, Syrigos KN, Zbar A, Sekara E, Michail P, Rosenberg T, Diamantis T. Clinical significance of serum levels of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in gastric cancer patients. Am J Gastroenterol. 2003;98:478–485. doi: 10.1111/j.1572-0241.2003.07259.x. [DOI] [PubMed] [Google Scholar]
- 21.Gogali A, Charalabopoulos K, Zampira I, Konstantinidis AK, Tachmazoglou F, Daskalopoulos G, Constantopoulos SH, Dalavanga Y. Soluble adhesion molecules E-cadherin, intercellular adhesion molecule-1, and E-selectin as lung cancer biomarkers. Chest. 2010;138:1173–1179. doi: 10.1378/chest.10-0157. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama M, Matsumura H, Shioda J, Aoki H, Nakamura H, Arakawa Y, Nirei K, Yamagami H, Kaneko M, Tanaka N, et al. Measurement of human intercellular adhesion molecule 1 in the blood is useful for predicting the occurrence of hepatocellular carcinomas from chronic hepatitis C and liver cirrhosis. Intervirology. 2006;49:327–338. doi: 10.1159/000095152. [DOI] [PubMed] [Google Scholar]
- 23.Kammerer S, Roth RB, Reneland R, Marnellos G, Hoyal CR, Markward NJ, Ebner F, Kiechle M, Schwarz-Boeger U, Griffiths LR, et al. Large-scale association study identifies ICAM gene region as breast and prostate cancer susceptibility locus. Cancer Res. 2004;64:8906–8910. doi: 10.1158/0008-5472.CAN-04-1788. [DOI] [PubMed] [Google Scholar]
- 24.Arandi N, Talei A, Erfani N, Ghaderi A. Intercellular adhesion molecule-1 genetic markers (+241G/A and +469A/G) in Iranian women with breast cancer. Cancer Genet Cytogenet. 2008;183:9–13. doi: 10.1016/j.cancergencyto.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Cox DG, Hankinson SE, Hunter DJ. Polymorphisms in the ICAM gene locus are not associated with breast cancer Risk. Cancer Epidemiol Biomarkers Prev. 2006;15:178–179. doi: 10.1158/1055-9965.EPI-05-0790. [DOI] [PubMed] [Google Scholar]
- 26.Vinceti M, Pellacani G, Casali B, Malagoli C, Nicoli D, Farnetti E, Bassissi S, Bergomi M, Seidenari S. High risk of cutaneous melanoma amongst carriers of the intercellular adhesion molecule-1 R241 allele. Melanoma Res. 2006;16:93–96. doi: 10.1097/01.cmr.0000198450.19204.dd. [DOI] [PubMed] [Google Scholar]
- 27.Howell WM, Rose-Zerilli MJ, Theaker JM, Bateman AC. ICAM-1 polymorphisms and development of cutaneous malignant melanoma. Int J Immunogenet. 2005;32:367–373. doi: 10.1111/j.1744-313X.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang QL, Li BH, Liu B, Liu YB, Liu YP, Miao SB, Han Y, Wen JK, Han M. Polymorphisms of the ICAM-1 exon 6 (E469K) are associated with differentiation of colorectal cancer. J Exp Clin Cancer Res. 2009;28:139. doi: 10.1186/1756-9966-28-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimura M, Obayashi H, Maruya E, Ohta M, Tegoshi H, Fukui M, Hasegawa G, Shigeta H, Kitagawa Y, Nakano K, et al. Association between type 1 diabetes age-at-onset and intercellular adhesion molecule-1 (ICAM-1) gene polymorphism. Hum Immunol. 2000;61:507–510. doi: 10.1016/s0198-8859(00)00101-4. [DOI] [PubMed] [Google Scholar]
- 30.International Union Against Cancer (UICC) TNM classification of malignant tumours. 7th ed. Sobin LH, Gospodarowicz MK, Wittekind Ch, editors. New York: Wiley; 2009. pp. 73–77. [Google Scholar]
- 31.Theodoropoulos G, Papaconstantinou I, Felekouras E, Nikiteas N, Karakitsos P, Panoussopoulos D, Lazaris ACh, Patsouris E, Bramis J, Gazouli M. Relation between common polymorphisms in genes related to inflammatory response and colorectal cancer. World J Gastroenterol. 2006;12:5037–5043. doi: 10.3748/wjg.v12.i31.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515–G520. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- 33.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 34.Schröder C, Witzel I, Müller V, Krenkel S, Wirtz RM, Jänicke F, Schumacher U, Milde-Langosch K. Prognostic value of intercellular adhesion molecule (ICAM)-1 expression in breast cancer. J Cancer Res Clin Oncol. 2011;137:1193–1201. doi: 10.1007/s00432-011-0984-2. [DOI] [PubMed] [Google Scholar]
- 35.Hayes SH, Seigel GM. Immunoreactivity of ICAM-1 in human tumors, metastases and normal tissues. Int J Clin Exp Pathol. 2009;2:553–560. [PMC free article] [PubMed] [Google Scholar]
- 36.Sadaria MR, Meng X, Fullerton DA, Reece TB, Shah RR, Grover FL, Weyant MJ. Secretory phospholipase A2 inhibition attenuates intercellular adhesion molecule-1 expression in human esophageal adenocarcinoma cells. Ann Thorac Surg. 2011;91:1539–1545. doi: 10.1016/j.athoracsur.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Hamaï A, Meslin F, Benlalam H, Jalil A, Mehrpour M, Faure F, Lecluse Y, Vielh P, Avril MF, Robert C, et al. ICAM-1 has a critical role in the regulation of metastatic melanoma tumor susceptibility to CTL lysis by interfering with PI3K/AKT pathway. Cancer Res. 2008;68:9854–9864. doi: 10.1158/0008-5472.CAN-08-0719. [DOI] [PubMed] [Google Scholar]
- 38.Rae JM, Cordero KE, Scheys JO, Lippman ME, Flockhart DA, Johnson MD. Genotyping for polymorphic drug metabolizing enzymes from paraffin-embedded and immunohistochemically stained tumor samples. Pharmacogenetics. 2003;13:501–507. doi: 10.1097/00008571-200308000-00008. [DOI] [PubMed] [Google Scholar]


