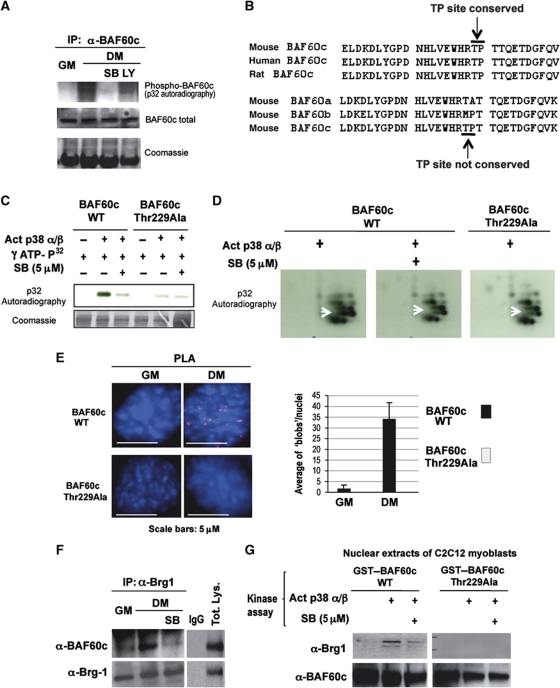
Figure 3.
BAF60c phosphorylation by p38α/β kinases mediates Brg1 recruitment. (A) In-vivo 32P labelling of C2C12 cells induced to differentiate (DM) in the presence or not of p38α/β inhibitor (SB) or Pi3K inhibitor (LY) was followed by immunoprecipitation of endogenous BAF60c and detection of 32P incorporation after SDS gel-electrophoresis. (B) The p38 consensus site was identified by sequence analysis of BAF60c using BioEdit software (Hall, 1997). A proline-directed threonine 229, suitable for p38 phosphorylation and conserved in BAF60c of mouse, rat and human is indicated with arrow (TP site). Note that the p38 consensus site is not present in BAF60a or BAF60b. (C) In-vitro kinase assay using active p38α and β as kinases, GST–BAF60c as a substrate and radioactive γ-32P-ATP in the presence or absence of SB. (D) The bands from the gel displayed in (C) were cut, eluted, trypsin digested and run on a two-dimensional gel. Arrow indicates a spot that disappears in the BAF60c wt in the presence of SB and in the BAF60c Thr229Ala mutant. (E) PLA was performed in C2C12 cells expressing Flag-tagged BAF60c wt or the BAF60c Thr229Ala mutant, using an anti-proline directed phospho-threonine antibody (mouse) and an anti-Flag antibody (rabbit). (F) Co-immunoprecipitation of endogenous BAF60c and Brg1 from C2C12 undifferentiated (GM) or differentiating (DM) myoblasts, in the presence or absence of the p38α and β kinase inhibitor SB. (G) GST pull-down assay with GST–BAF60c wt or Thr229Ala mutant, first incubated with p38α and β kinases in a buffer permissive for phosphorylation, in the presence or not of SB, and then incubated with nuclear extracts from C2C12 cells. The precipitated material was blotted with anti-Brg1 and BAF60c antibodies.