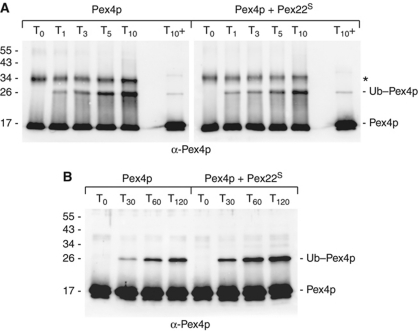
Figure 2.
Pex22p binding stimulates transfer of Ub to a substrate. (A) Pex415–183 charging assays, performed in the absence (left panel) or presence (right panel) of Pex22S, with protein concentrations as follows: 0.27 μM E1, 29 μM Ub, 10 mM ATP, 8.3 μM Pex415–183 and 11 μM Pex22S. Charging of E2 was assessed by following the formation of thioester-linked Ub–Pex415–183. Reactions were quenched in non-reducing loading buffer at the indicated times, subjected to SDS–PAGE and probed with anti-Pex4p antibodies. To gauge background levels of lysine-linked Ub–Pex415–183, samples taken after 10 min were also analysed after treatment with β-mercaptoethanol (T10+). *Represents a dimeric form of Pex4p visible when DTT is absent from the reaction. (B) E2 self-ubiquitination assay, with protein concentrations as in Figure 1, using the K48R form of Ub. Samples of reactions were taken at the time points indicated and subjected to SDS–PAGE, western blotting and anti-Pex4p staining. Pex22S binding is not involved in promoting the charging of Pex4p by E1 (A) but enhances the transfer of Ub to a substrate (B).