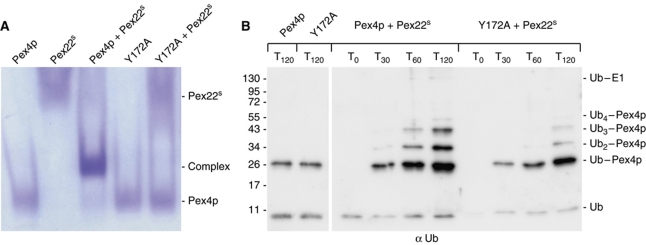
Figure 6.
In-vitro characteristics of the Y172A mutant form of Pex415–183. (A) Native gel electrophoresis analysis of the ability of wild-type and Y172A forms of Pex415–183 to bind Pex22S. (B) E2 self-ubiquitination assay performed as in Figure 2B and containing E1, ATP, wild-type or Y172A forms of Pex415–183 in the presence or absence of Pex22S. Samples of the reactions were taken at the time points indicated and probed with anti-Ub antibodies. The Y172A mutation, which disturbs Pex22S binding (A), does not affect the intrinsic activity of Pex415–183 (B, left panel) but inhibits the Pex22S-dependent enhancement of Pex4p activity (B, right panel).