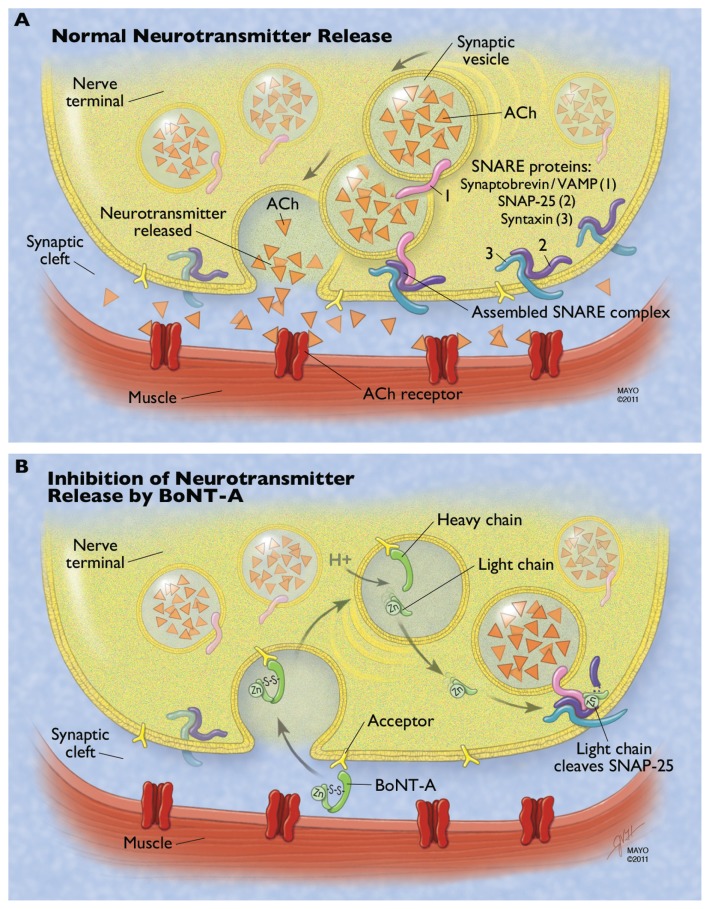
Figure 1.
Mechanism of botulinum neurotoxin type A (BoNT-A) at the neuromuscular junction. (A) Normal neurotransmitter release requires fusion of the vesicle membrane to the membrane of the presynaptic nerve terminal. This process is guided by the fusion of three proteins that make up the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex: the vesicle-associated membrane protein (VAMP or synaptobrevin) and the membrane-bound syntaxin and synaptosomeassociated protein of 25 kDa (SNAP-25). ACh is released from the vesicle, diffuses across the synaptic cleft, and binds to the acetylcholine (Ach) receptor, resulting in normal muscular contraction. (B) The heavy chain of BoNT-A binds to a ganglioside acceptor in the plasma membrane of the presynaptic nerve terminal. This leads to receptormediated endocytosis of the neurotoxin. The acidic environment of the synaptic vesicle or endosome leads to a conformational change in the toxin and eventual reduction of the linking disulfide bond, freeing the light chain. The light chain translocates to the cytosol and cleaves the C-terminal of the SNAP-25 protein. This inhibits SNARE complex formation and therefore inhibits neurotransmitter release.
Note: Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.