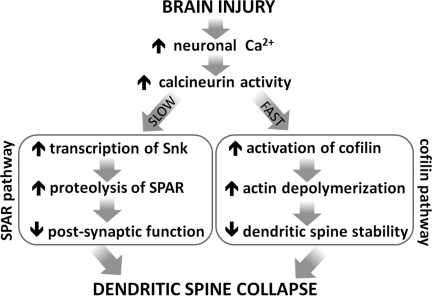
FIG. 10.
Calcineurin-dependent mechanisms by which brain injury could cause dendritic spine collapse. Brain injury can cause an increase in the activity of the calcium-sensitive phosphatase calcineurin (CaN). CaN-dependent signaling can then lead to dendritic spine collapse through different signaling pathways. In the neocortex, for example, CaN activity may lead to a rapid de-phosphorylation/activation of the actin-depolymerizing protein cofilin. Excessive cofilin activity could disrupt the spine's actin-rich cytoskeleton, resulting in spine shrinkage or de-stabilization (e.g., Zhou et al., 2004). In the hippocampus, however, a different mechanism may be involved, a mechanism involving CaN but not cofilin. For example, an injury-induced increase in CaN activity can lead to a transcriptional upregulation of serum-induced kinase (Snk), ultimately resulting in the targeted proteolysis of a spine-stabilizing protein, spine-associated Rap guanosine triphosphatase activating protein (SPAR; Pak and Sheng, 2003). SPAR loss is associated with degeneration of post-synaptic function and structure, including spine loss (Pak and Sheng, 2003; Pak et al., 2001; Seeburg et al., 2008). Therefore, cofilin activation and SPAR proteolysis represent different mechanisms by which CaN may cause spine loss. Evidence from the present study favors cofilin activation as a regional mechanism of spine loss after TBI, but does not rule out focal involvement of SPAR proteolysis.