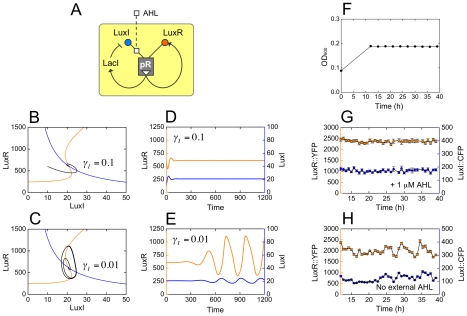
Figure 7. Oscillations in the dual positive/negative-feedback system.
(A) In the dual feedback system, both LuxR as well as the LacI repressor are placed downstream of pR. LuxR positively regulates its own expression; LacI negatively regulates the expression of LuxI via the pLac promoter. We model the system using measured PLF parameter values (Table S3), as well as additional parameters describing LacI-pLac interactions and protein decay rates whose values are chosen in order to generate oscillations; the decay rate 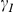 of LuxI is left as a free parameter (Supporting Information, Text S1: The dual positive/negative-feedback system). (B,C) Numerical phase plane analysis. The orange curve is the LuxR nullcline along which
of LuxI is left as a free parameter (Supporting Information, Text S1: The dual positive/negative-feedback system). (B,C) Numerical phase plane analysis. The orange curve is the LuxR nullcline along which 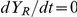 ; the blue curve is the LuxI nullcline along which
; the blue curve is the LuxI nullcline along which 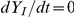 ; the intersection of these curves is a fixed point which could be stable or unstable. The black curve is shows the system trajectory, which runs counterclockwise as time progresses. (B) For
; the intersection of these curves is a fixed point which could be stable or unstable. The black curve is shows the system trajectory, which runs counterclockwise as time progresses. (B) For 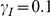 the fixed point is stable, and the system fails to oscillate. (C) For
the fixed point is stable, and the system fails to oscillate. (C) For 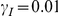 the fixed point is unstable, and the system enters a limit-cycle oscillation. (D,E) We show LuxR (orange, left axis) and LuxI (blue, right axis) values as functions of time (in arbitrary units), corresponding to the trajectories from (B,C). (F) Our experiments are conducted in a nitrogen-limited chemostat, which maintains a steady-state cell density of OD600 = 0.185. (G,H) Measured response of dual feedback cells in the chemostat. Datapoints represent the mean fluorescence values of LuxR::YFP (orange, left axis) and LuxI::CFP (blue, right axis) for a population of ∼500 cells; errorbars represent standard error of means. (G) In the control experiment cells are grown in the presence of 1 µM AHL, thus abolishing negative feedback, and the system settles into a steady state. Note that panels (D) and (G) represent very different steady-state situations, and should not be directly compared. (H) In the oscillation experiment cells are initially primed with 1 µM AHL, but this is allowed to dilute out from the 12 h timepoint. From about 20 h, the system displays oscillations that are synchronized over the entire population and stable for 15 h, in qualitative agreement with the numerical predictions.
the fixed point is unstable, and the system enters a limit-cycle oscillation. (D,E) We show LuxR (orange, left axis) and LuxI (blue, right axis) values as functions of time (in arbitrary units), corresponding to the trajectories from (B,C). (F) Our experiments are conducted in a nitrogen-limited chemostat, which maintains a steady-state cell density of OD600 = 0.185. (G,H) Measured response of dual feedback cells in the chemostat. Datapoints represent the mean fluorescence values of LuxR::YFP (orange, left axis) and LuxI::CFP (blue, right axis) for a population of ∼500 cells; errorbars represent standard error of means. (G) In the control experiment cells are grown in the presence of 1 µM AHL, thus abolishing negative feedback, and the system settles into a steady state. Note that panels (D) and (G) represent very different steady-state situations, and should not be directly compared. (H) In the oscillation experiment cells are initially primed with 1 µM AHL, but this is allowed to dilute out from the 12 h timepoint. From about 20 h, the system displays oscillations that are synchronized over the entire population and stable for 15 h, in qualitative agreement with the numerical predictions.