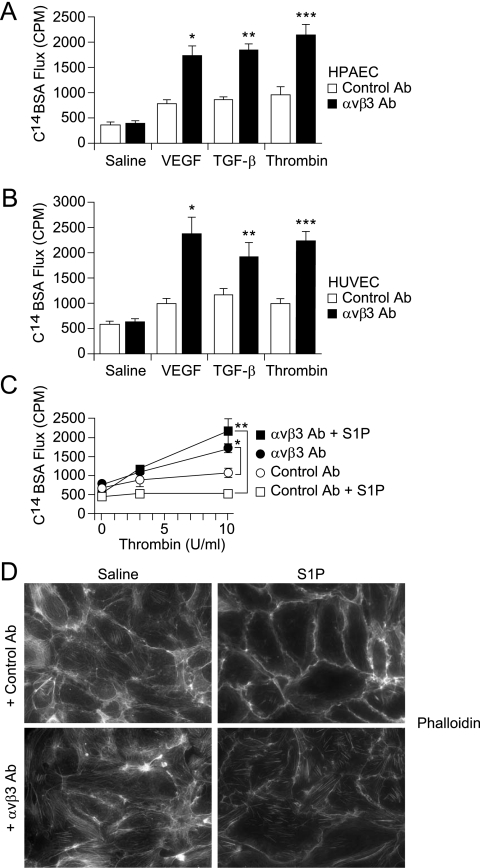
Figure 3.
(A and B) αvβ3 antibodies increase the endothelial permeability response to inflammatory agonists. Serum-starved confluent human pulmonary artery endothelial cell (HPAEC) (A) and human umbilical vein endothelial cell (HUVEC) (B) monolayers were incubated with αvβ3 or control antibodies (Ab) (10 μg/ml, 1 h) before stimulation with vascular endothelial growth factor (VEGF) (30 ng/ml, 10 min), transforming growth factor (TGF)-β (10 ng/ml, 10 min), or thrombin (10 U/ml, 5 min). C14–bovine serum albumin (BSA) flux across monolayers was measured as counts per minute (CPM) from media collected from basolateral wells. (A) HPAECs: Data shown are the means ± standard errors, n = 4 samples per group. Control versus αvβ3 Ab: P = 0.736 for saline, *P = 0.001 for VEGF, **P = 0.001 for TGF-β, ***P = 0.001 for thrombin. (B) HUVECs: Data shown are the means ± standard errors, n = 4 for each group. Control versus αvβ3 Ab: P = 0.612 for saline, *P = 0.002 for VEGF, **P = 0.032 for TGF-β, ***P = 0.002 for thrombin. (C) αvβ3 antibodies inhibit sphingosine-1 phosphate (S1P)–induced resistance to increased permeability effects of thrombin. Serum-starved confluent HPAEC monolayers were incubated with αvβ3 or isotype control antibodies (10 μg/ml, 1 h) before stimulation with S1P (0.5 μM, 5 min) or thrombin (10 U/ml, 5 min). C14-BSA flux across monolayers was measured as CPM collected into basolateral wells. Data shown are the means ± standard errors, n = 4 samples per group. *P = 0.0006 for thrombin-treated: control versus αvβ3 Ab. **P = 0.0007 for S1P- and thrombin-treated: control versus αvβ3 Ab. (D) αvβ3 antibodies disrupt S1P-induced cortical actin in favor of stress fiber formation. Confluent monolayers of HPAECs were pretreated with control or αvβ3 antibodies, then stimulated with S1P or saline control. Cells were then fixed, permeabilized, and stained with rhodamine-phalloidin. Images were acquired with a ×40 dry objective.