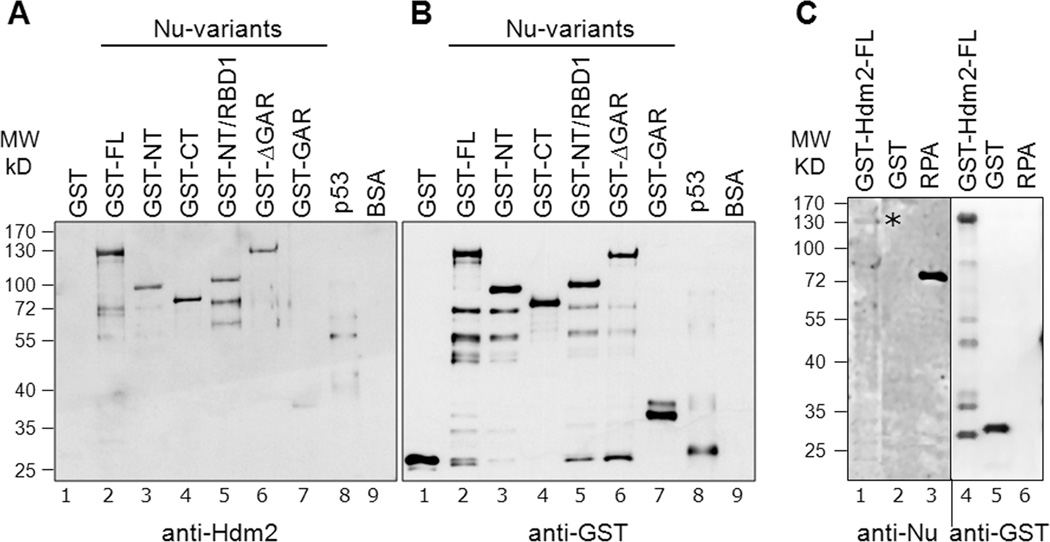
Fig. 2. Elucidation of direct nucleolin-Hdm2 interactions and identification of Hdm2-interacting domains on nucleolin.
A, Equivalent amounts (400 ng) of GST-tagged nucleolin FL (lane 2) or nucleolin domains (lanes 3 to 7) were subjected to SDS-PAGE. As negative controls for the nucleolin-Hdm2 interaction, GST alone (lane 1) and BSA (lane 9) were used. Cleaved p53 (after removal of GST-tag, lane 8) served as a positive control. The separated proteins were then electrotransferred to a nitrocellulose membrane for Far-Western analysis. The membrane was probed with purified Hdm2 protein (0.2 µg/ml), as described in Materials and Methods, and the bound Hdm2 detected by a monoclonal anti-Hdm2 antibody (SMP14). B, Equivalent loading of the GST-nucleolin fusion proteins was confirmed by running a parallel blot and probing directly with anti-GST antibodies. The multiple lower molecule bands visualized could be attributed to self-cleaving properties of nucleolin at the C-terminus [42,43]. These data indicate that both the nucleolin NT and RBD domains can independently bind Hdm2. C, Nucleolin interaction with Hdm2 in reciprocal Far-Western. Equivalent amounts (400 ng) of GST-tagged Hdm2 FL (lane 1), GST (lane 2), and non-GST-tagged RPA (lane 3) were subjected to SDS-PAGE and then electrotransferred to a nitrocellulose membrane for subsequent Far-Western analysis. The membrane was probed with purified nucleolin FL (isolated from yeast with a GST tag), and the bound nucleolin was then detected using an anti-nucleolin antibody (MS-3). The location of bound nucleolin is denoted with an asterisk. Equal loading of GST and GST-Hdm2 FL was confirmed by running a parallel blot, and probing directly for anti-GST antibodies (RPA is not GST-tagged and hence not recognized by anti-GST). Although each three lane group was originally present on the same blot, they were spliced adjacent to each other for this figure.