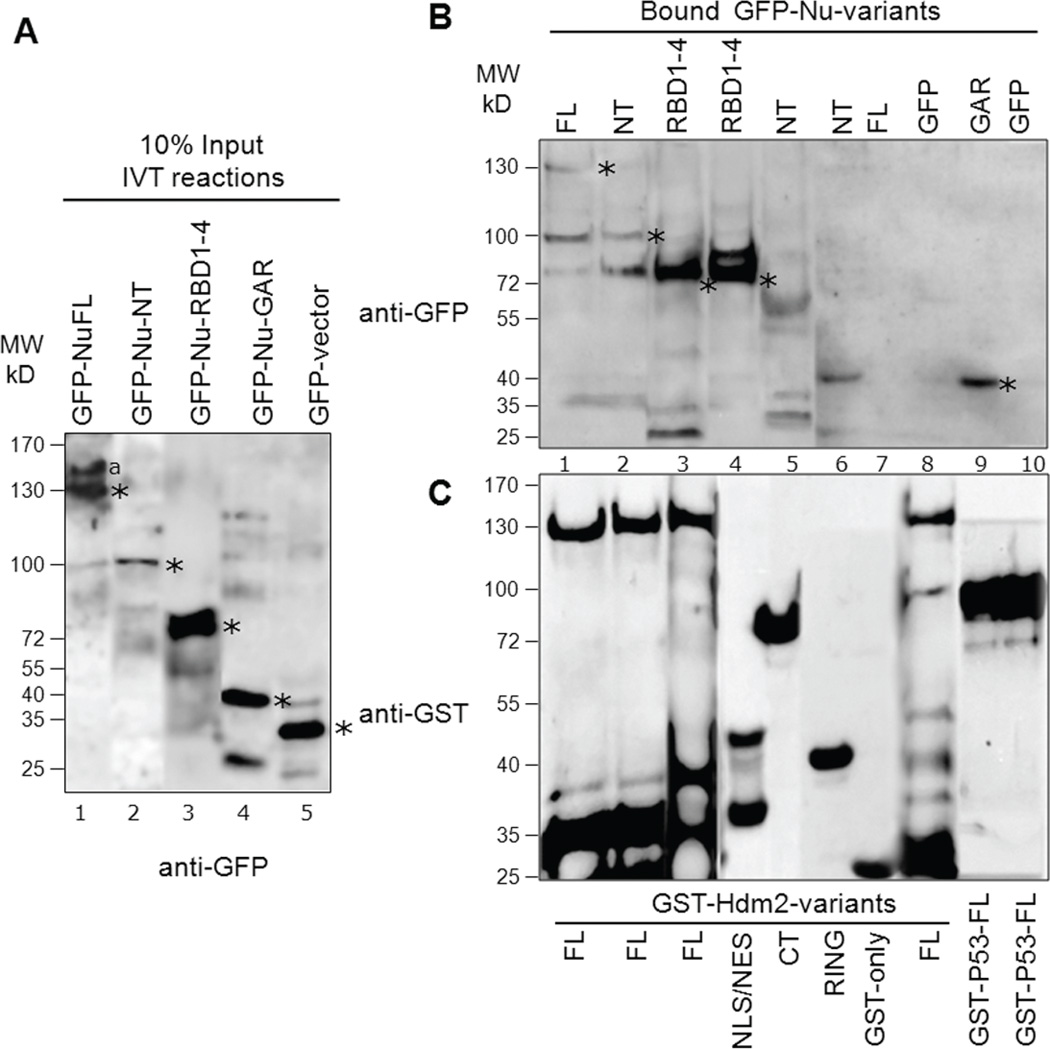
Fig. 6. Nucleolin directly interacts with Hdm2 and p53 using distinct domains in vitro.
In vitro translated (IVT) GFP-tagged proteins (nucleolin variants or GFP alone) were incubated with purified GST-tagged proteins (Hdm2, p53 or GST alone) to test association. A, A representative blot indicating the translated GFP-nucleolin variants: 10% of input levels used in binding reactions shown in panel B were analyzed by Western for GFP. We repeatedly observed lesser IVT product for nucleolin-NT (lane 2). The location of expected molecular size of each nucleolin-variant and GFP is denoted with an asterisk. The higher molecular weight product (lane 1, denoted by “a”) could be due to partial translational read-through of the normal mRNA stop codon during the IVT reaction. Lower molecular bands observed could be due to proteolysis of the translated product. B, Identification of nucleolin domains that interact with Hdm2 and p53: Bound fractions from binding reactions were analyzed by SDS-PAGE and Western blotting using anti-GFP. GFP-Nu-FL, NT and RBD1–4 directly interact with GST-Hdm2-FL (lanes 1–3). RBD1–4 also strongly interacts with GST-Hdm2 NLS/NES domain (lane 4) while nucleolin-NT domain does not interact with either Hdm2-CT or RING domain (lanes 5–6). As negative controls, the translated GFP-nucleolin-FL did not interact with GST alone (lane 7), nor did GFP significantly bind to GST-Hdm2-FL (lane 8). On the other hand, nucleolin-GAR domain strongly interacts with GST-p53-FL (lane 9) while no interaction was detected for the negative control GFP with p53 (lane 10). Detectable binding of nucleolin-variants to Hdm2-FL or p53-FL are indicated by asterisks. C, The blots were subsequently stripped and reprobed with anti-GST to reveal the loading of the purified GST-tagged proteins in the corresponding binding reaction. The lower molecular weight products observed indicate that some proteolysis of translated products (blot B) or GST-tagged constructs (blot C) occur, despite of the presence of protease inhibitor in the binding reactions. After scanning, the particular sections were cropped and rearranged for easier display.