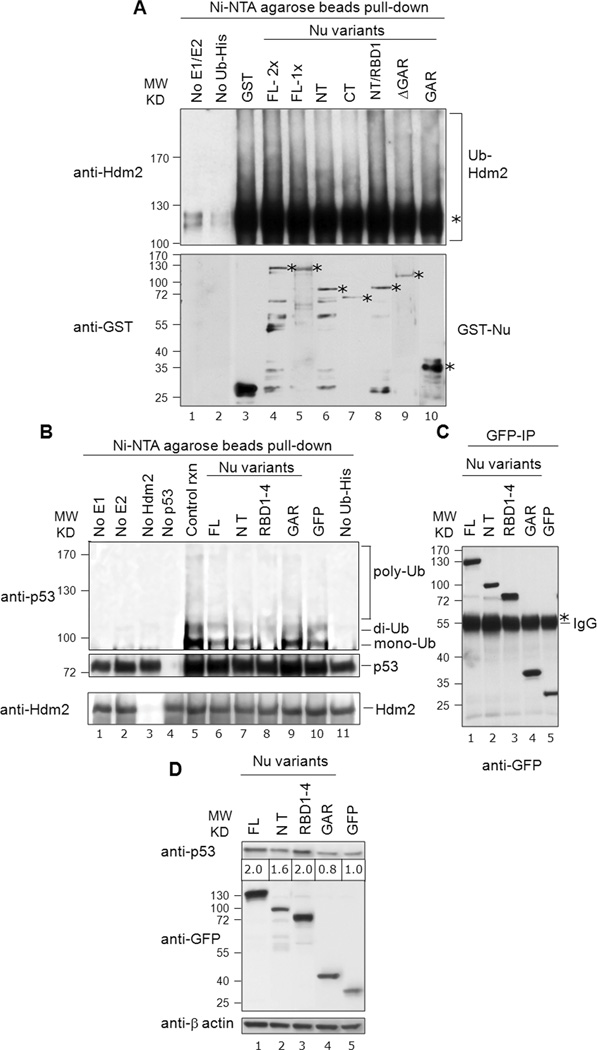
Fig. 7. Nucleolin RBD1–4 selectively inhibits p53 ubiquitination in vitro, but not Hdm2 auto-ubiquitination.
A, Nucleolin does not significantly alter Hdm2 auto-ubiquitination: Nucleolin FL or truncation domains (150 ng; 1×) were analyzed for their effects on Hdm2 auto-ubiquitination (upper panel, lanes 4 to 10), using His-tagged ubiquitin. Reaction products were purified on Ni-NTA-agarose beads (i.e., to isolate the ubiquitinated products), and then subjected to SDS-PAGE and Western analysis for Hdm2. The mono-ubiquitinated Hdm2 is marked with an asterisk. Control reactions lacked the E1/E2 ubiquitination factors (lane 1) or Ub-His (lane 2). These data indicate that nucleolin does not significantly alter Hdm2 auto-ubiquitination. To visualize GST-tagged nucleolin proteins, a parallel blot was prepared and probed directly for anti-GST antibodies (lower panel). The location of expected molecular size of each nucleolin-variant and GST is denoted with an asterisk. B, Nucleolin RBD1–4 significantly inhibits p53-ubiquitination: Nucleolin FL (40 ng) or truncation domains were added to ubiquitination reactions including p53. The same blot subsequently stripped and reprobed with anti-Hdm2 to reveal the equivalent loading of the purified Hdm2 in the corresponding reaction (bottom panel). Various controls are also included, as indicated (lanes 1 to 5, 10 and 11). C, Immunoprecipitated GFP-nucleolin fusion proteins on IgG beads, utilized in corresponding in vitro p53-ubiquitination reactions were visualized on a parallel blot after probing with anti-GFP antibodies. The heavy band of IgG is also visible (marked with an asterisk). D, Effects of nucleolin domain expression on p53 levels: Constructs expressing various GFP-tagged nucleolin domains were transfected into U2OS cells. Post-transfection (36 h), endogenous p53 was detected by Western using an anti-p53 (DO-1) antibody. GFP blot indicated the expression levels of each nucleolin domain while actin served as a loading control. Note that the level of p53 protein (denoted below the p53 Western blot) was normalized to β-actin expression, after quantitation with Image J software (NIH). We observe a dose-dependent effect of expression of nucleolin protein and p53 stabilization with the range of 1.2 to 2.0-fold increase in p53 protein levels (compare with Fig. S3).