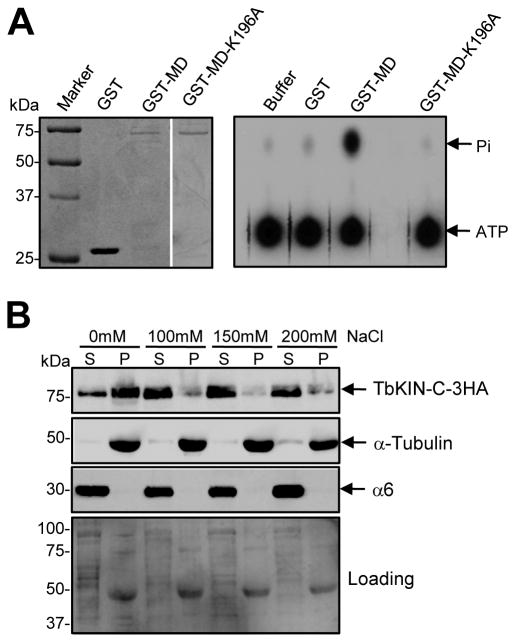
Figure 1. TbKIN-C possesses in vitro ATPase activity and associates with tubulin microtubules in vivo.
(A). In vitro ATPase activity assay of the motor domain of TbKIN-C. The motor domain of TbKIN-C (MDTbKIN-C) and its K196A mutant (MD-KATbKIN-C) were purified as GST-fusion proteins from E. coli (left panel) and used for in vitro ATPase assays (right panel). Purified GST protein was included as a control. (B). In vivo association of TbKIN-C with tubulin microtubules in trypanosomes. 3HA-tagged TbKIN-C in soluble fraction and insoluble cytoskeleton fraction of trypanosome cells were detected by Western blot with anti-HA antibody. The same blot was also probed with anti-α-tubulin antibody and anti-α6 protein, a subunit of the 26S proteasome. S: supernatant; P: pellet.