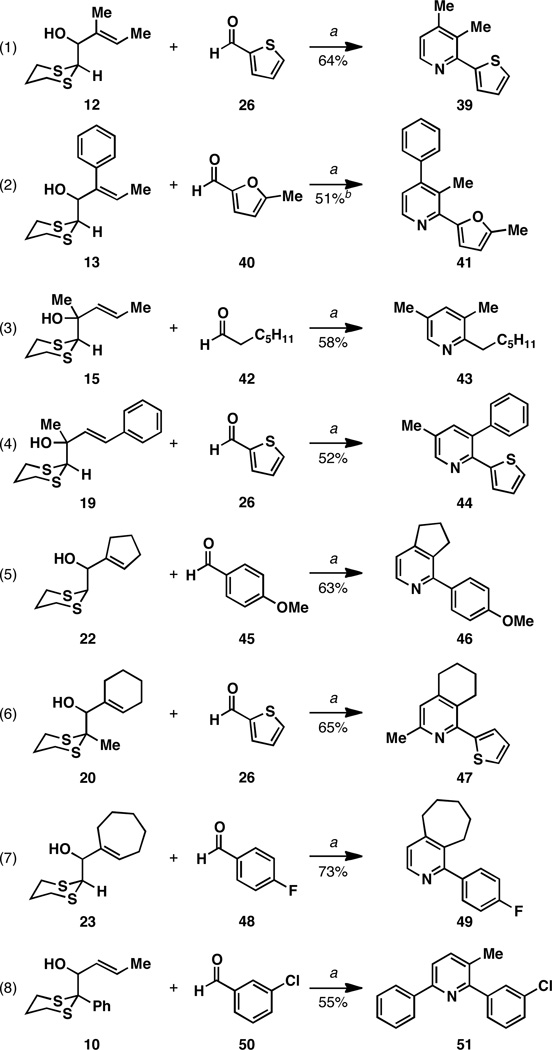
Figure 8.
Exploring the scope of this metallacycle-mediated approach to de novo pyridine synthesis. Reaction conditions: (a) Timediated coupling – aldehyde (2 eq), LiHMDS, Et2O, then Ti(Oi-Pr)4, c-C5H9MgCl or n-BuLi, then add Li-alkoxide of allylic alcohol (1 eq); cyclization – HgO, BF3•OEt2, HCl, THF (100 °C); (b) AgNO3 was employed for the cyclization step: AgNO3, THF, H2O (60 °C).