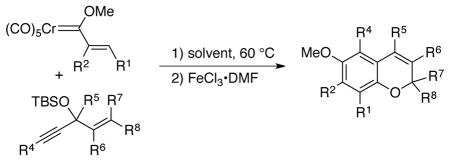
Table 2.
Chromene Synthesis via Carbene Complexes and Alkoxyenynes.a
 | ||
|---|---|---|
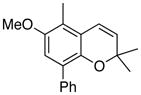 23 69% (CH2Cl2) 66% (CH3CN) |
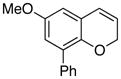 24 47% (CH3CN) |
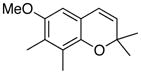 22 88% (CH2Cl2) 65% (CH3CN) |
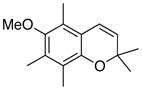 25 84% (CH2Cl2) 87% (CH3CN) |
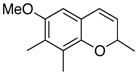 26 70% (CH2Cl2) |
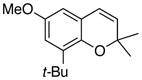 27 78% (CH2Cl2) 78% (CH3CN) |
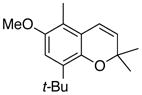 28 74% (CH2Cl2) 86% (CH2Cl2) b 65% (CH3CN) c,d |
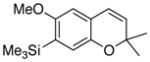 29 56% (CH2Cl2) 23% (CH3CN) |
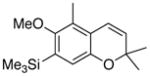 30 84% (CH2Cl2) 35% (CH3CN) |
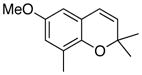 31 41% (CH2Cl2) 83% (CH3CN) c |
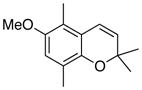 32 61% (CH2Cl2) 48% (CH3CN) c |
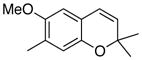 33 45% (CH2Cl2) 26% (CH3CN) 55% (hexane) |
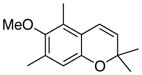 34 68% (CH2Cl2) 34% (CH3CN) 74% (hexane) |
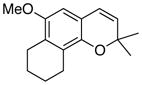 35 72% (CH2Cl2) 65% (CH3CN) c |
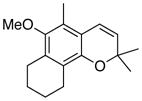 36 80% (CH2Cl2) 68% (CH3CN) c |
Unless otherwise specified, all reactions were run at 0.03 M in carbene complex in the indicated solvent with 1.2 equiv of the enyne at 60 °C for 24 h. If oxidation was used it was carried out with 7.5 equiv of FeCl3•DMF complex. All yields are isolated yields after chromatography on silica gel.
Reaction performed with 10 equiv of aniline.
Oxidative workup not used.
Isolation after treatment of crude reaction mixture with trifluoromethane sulfonic acid.
