Fig. 3. Comparison of AphB with other LysR-type regulators.
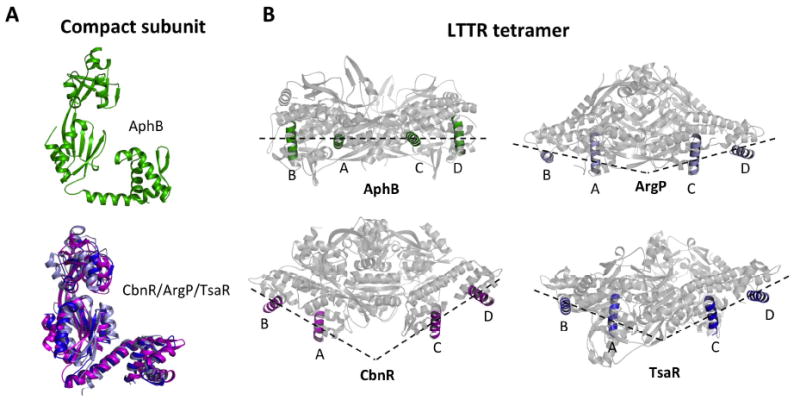
(A) The AphB wild-type (green) compact subunit showing that the DNA-binding and regulatory domains are located on the same side of the linker helix (top). Superposition of the linker helices of the compact subunits of ArgP (silver) and TsaR (blue) onto that of CbnR (magenta), showing that the DNA-binding and regulatory domains are located on opposite sides of the linker helix (bottom). The RD of CbnR was first superposed with that of AphB WT, in the same orientation as seen at top, to maintain the same perspective. (B) The recognition helices of the AphB tetramer (green) fall on a single plane and form a flat DNA-binding interface whereas the recognition helices of CbnR (magenta), ArgP (silver) and TsaR (blue) form a V-shaped DNA-binding interface. AphB was rotated 90° from the orientation shown in Fig. 2 and then the CbnR, ArgP and TsaR recognition helices were superposed with those of AphB.
