Abstract
PURPOSE
Depression commonly accompanies diabetes, resulting in reduced adherence to medications and increased risk for morbidity and mortality. The objective of this study was to examine whether a simple, brief integrated approach to depression and type 2 diabetes mellitus (type 2 diabetes) treatment improved adherence to oral hypoglycemic agents and antidepressant medications, glycemic control, and depression among primary care patients.
METHODS
We undertook a randomized controlled trial conducted from April 2010 through April 2011 of 180 patients prescribed pharmacotherapy for type 2 diabetes and depression in primary care. Patients were randomly assigned to an integrated care intervention or usual care. Integrated care managers collaborated with physicians to offer education and guideline-based treatment recommendations and to monitor adherence and clinical status. Adherence was assessed using the Medication Event Monitoring System (MEMS). We used glycated hemoglobin (HbA1c) assays to measure glycemic control and the 9-item Patient Health Questionnaire (PHQ-9) to assess depression.
RESULTS
Intervention and usual care groups did not differ statistically on baseline measures. Patients who received the intervention were more likely to achieve HbA1c levels of less than 7% (intervention 60.9% vs usual care 35.7%; P <.001) and remission of depression (PHQ-9 score of less than 5: intervention 58.7% vs usual care 30.7%; P <.001) in comparison with patients in the usual care group at 12 weeks.
CONCLUSIONS
A randomized controlled trial of a simple, brief intervention integrating treatment of type 2 diabetes and depression was successful in improving outcomes in primary care. An integrated approach to depression and type 2 diabetes treatment may facilitate its deployment in real-world practices with competing demands for limited resources.
Keywords: Medication adherence, type 2 diabetes mellitus, depression, comorbidity, chronic disease, primary health care, randomized controlled trial
INTRODUCTION
A bidirectional association has been found between depression and diabetes mellitus.1 Depression is a risk factor for diabetes,2 and diabetes increases risk for the onset of depression.3 Not only is depression common in patients with diabetes, it also contributes to poor adherence to medication and dietary regimens, physical inactivity, poor glycemic control, reduced quality of life, disability, and increased health care expenditures.4–9
The purpose of this study was to carry out a randomized controlled trial to test the effectiveness of integrated care management of type 2 diabetes mellitus (type 2 diabetes) and depression in comparison with usual care services in primary care. Several studies have shown that a variety of primary care interventions can improve diabetes10 and depression outcomes.11 Few of these interventions are being implemented in practice, however.12,13 Integrated care is needed to enhance quality of care, quality of life, consumer satisfaction, and system efficiency for patients with complex, long-term problems cutting across multiple services, clinicians, and settings.14
We chose an adherence-based approach because, although efficacious pharmacotherapy for many chronic medical conditions exists, many patients are not adherent to treatment and therefore are at increased risk for a variety of complications.15 Poor adherence to treatment remains a major impediment to improving care, particularly among patients with comorbid diabetes and depression.16 Compared with patients who are not depressed, depressed patients who have diabetes are more likely to be nonadherent to medication regimens17,18 and exhibit worsening diabetes management.19,20 The management of comorbid depression and diabetes should be integrated and tailored for preference, tolerance, and simplicity to enhance adherence to prescribed medical regimens.4
A review of the literature found only 2 randomized controlled trials integrating care for the management of depression with diabetes.21 Katon and colleagues at Group Health Cooperative, a nonprofit health maintenance organization in Seattle, Washington, tested an intensive intervention for adults with major depression and poorly controlled diabetes and/or coronary heart disease carried out by an advanced-practice nurse; they found the intervention significantly improved control of medical disease and depression.22 Partners Healthcare, a nonprofit integrated health care system in Boston, Massachusetts, is testing an intensive intervention for adults with poorly controlled diabetes and major depression or dysthymia carried out by a master’s trained therapist, but the data have yet to be published. In contrast to the Group Health Cooperative and Partners Healthcare studies, our study was conducted in community-based primary care practices and assessed a brief, simple intervention with a focus on improving adherence that was specifically developed for patients with type 2 diabetes mellitus. Our study involves an interventionist who acts as an intermediary or liaison between the depressed patient with type 2 diabetes and the physician in promoting type 2 diabetes and depression treatment and patient adherence. We hypothesized that in a sample of primary care patients with depression and type 2 diabetes, patients who were randomized to receive the intervention compared with usual care would show the following after a 3-month period: (1) fewer depressive symptoms, (2) lower glycated hemoglobin (HbA1c) levels, (3) a greater proportion of patients who had 80% or greater adherence to an antidepressant, and (4) a greater proportion of patients who had 80% or greater adherence to an oral hypoglycemic agent.
METHODS
Recruitment Procedures
Patients were recruited from 3 primary care practices in Philadelphia, Pennsylvania. The protocol was approved by the University of Pennsylvania Institutional Review Board. From April 2010 to April 2011, patients who had a diagnosis of type 2 diabetes mellitus, a prescription for an oral hypoglycemic agent within the past year, and a prescription for an oral antidepressant within the past year were identified by means of an electronic health record. Identified patients with an upcoming appointment were approached for further screening. The inclusion criteria were (1) aged 30 years and older, (2) a diagnosis of type 2 diabetes and a current prescription for an oral hypoglycemic agent, and (3) a current prescription for an antidepressant. We chose to include patients with a range of depressive symptoms reflecting the relapsing, remitting nature of depression in primary care.23 The age cutoff was chosen because of its importance in the detection, screening, and intervention for diabetic patients.24 Exclusion criteria were (1) inability to give informed consent, (2) cognitive impairment at baseline (Mini-Mental State Examination [MMSE] less than 21),25 (3) residence in a care facility that provides medications on schedule, and (4) unwillingness or inability to use the Medication Event Monitoring System (MEMS), a system in which microelectronic monitors on pill bottles provide the precise date and time of container opening.
Study Design
This trial consisted of 2 phases: a run-in phase and a randomized controlled trial phase. The purpose of the 2-week run-in phase was to collect preintervention adherence rates for all patients. During this phase data were also collected on demographic characteristics, blood pressure, low-density lipoprotein (LDL) cholesterol levels, body mass index (BMI), depressive symptoms, and HbA1c levels. No intervention was performed during this phase. Following completion of the 2-week run-in phase, patients entering phase 2 of the study were randomized within each practice by flip of a coin to either the integrated care intervention or usual care. Physicians were told which patients were enrolled in the integrated care intervention to allow for collaboration with the integrated care manager, but they were blinded to enrollment in the usual care group.
Intervention
We carried out an integrated care intervention in which the integrated care manager collaborated with physicians to offer education and guideline-based treatment recommendations to patients and to monitor adherence and clinical status. The key components of this intervention were the provision of an individualized program to improve adherence to antidepressants and oral hypoglycemic agents that recognizes patients’ social and cultural context, and the integration of depression treatment with type 2 diabetes management. The integrated care manager worked individually with patients to address the factors involved in adherence presented in our conceptual model, adapted from Cooper and colleagues,26 and previously published.27,28 In our conceptual model, the patient-level factors resulting in nonadherence included depression, chronic medical conditions, function, cognition, social support, cost of medications, side effects, and past experiences with medications. Patient-level factors were addressed through a variety of activities that included in-person sessions, telephone contacts, and collaborating with the physician. Through in-person sessions and telephone conversations, the integrated care manager provided education about depression and type 2 diabetes, emphasizing the importance of controlling depression to manage diabetes; help to identify target symptoms; explanations for the rationale for antidepressant and oral hypoglycemic agent use; assessment for side-effects and assistance in their management; assessment for progress (eg, reduction in depressive symptoms and improvement in finger stick results); assistance with referrals; and monitoring and response to life-threatening symptoms (eg, chest pain, suicidal thoughts and actions).
The intervention was presented to patients as a supplement to, rather than a replacement for, existing primary care treatment. We chose this multifaceted approach because education alone has not been found to be effective for improving adherence.29 The intervention consisted of 3, 30-minute in-person sessions (at baseline, 6 weeks, and 12 weeks) and 2, 15-minute telephone-monitoring contacts over a 3-month period. Two research coordinators (1 master’s level and 1 bachelor’s level) were trained as integrated care managers and administered all intervention activities. Before trial initiation, the integrated care managers received training on pharmacotherapy for depression and type 2 diabetes management during weekly clinical sessions with the principal investigator. (A summary of the intervention is available in the Supplemental Appendix, at http://www.annfammed.org/content/10/1/15/suppl/DC1.) We conducted a pilot study among 58 participants.28
Usual Care
At baseline, 6, and 12 weeks, patients in the usual care group underwent the same assessments as patients in the integrated care intervention. Assessments were conducted in person (as were assessments in the intervention group). Research assistants who conducted all assessments were blinded to patient’s randomization status.
Measurement Strategy
Potential study patients were screened for cognitive impairment using the MMSE, a short standardized mental status examination widely used for clinical and research purposes.30 Patients were asked whether they resided in a care facility that provided medications on schedule and whether they were unwilling or unable to use MEMS. At baseline sociodemographic characteristics were assessed using standard questions. Blood pressure was assessed in accordance with American Heart Association Guidelines.31 BMI was calculated as weight in kilograms divided by the square of height in meters.32 LDL cholesterol was obtained using standard laboratory techniques in accordance with National Heart Lung and Blood Institute Expert Panel Guidelines.33 Functional status was measured using the Medical Outcomes Study Short Form (SF-36).34 Adherence to antidepressants and oral hypoglycemic agents was measured during the 2-week run-in phase and at 6 and 12 weeks using electronic-monitoring data obtained from MEMS caps.
At baseline and 12 weeks blood glycemic control was assessed in accordance with American Diabetes Association Guidelines.35 HbA1c assays were performed using the in2it A1C Analyzer (Bio-Rad Laboratories, Hercules, California). Point-of-care testing using this device has acceptable precision and agreement in comparison with laboratory services.36 Depressive symptoms were measured using the 9-item Patient Health Questionnaire (PHQ-9) at baseline, 6, and 12 weeks.
Analytic Strategy
We compared baseline characteristics of patients in the integrated care intervention with baseline usual care patient characteristics using the t test and Fisher’s exact test (for continuous or categorical variables as appropriate). In addition, characteristics of patients who participated and who refused were compared using t tests and Fisher’s exact tests. Analysis proceeded at the patient level, and data were analyzed according to the treatment to which patients were randomized (intent-to-treat). Practice site was included in the models to account for potential clustering by practice. The models were adjusted for baseline measures (HbA1c or PHQ-9). We used repeated measures linear models in which mean response (eg, HbA1c and depressive symptoms) depends on the covariates of interest (treatment assignment and time since randomization) and an unstructured variance-covariance matrix to account for the extra correlation within individual patients. The parameter of interest was the time by treatment interaction, which represents the relative difference in change over time among the patients assigned to the intervention group compared with patients assigned to the usual care group. We contrasted the expected value of the outcome in each treatment group at 12 weeks with the value at baseline, the time of randomization. The intervention effect was measured as the difference between the 12-week effect in the treatment group and the 12-week effect in the usual care group.
We also considered categorical versions of the type 2 diabetes and depression outcomes. As recommended by clinical guidelines, we calculated whether a patient achieved an HbA1c level of less than 7% at 12 weeks.35 Depression remission was defined by a PHQ-9 score of less than 5 at follow-up.37 We used logistic regression to model the categorical diabetes outcome and repeated measures logistic regression to model depression remission. For both models, we report the odds ratio and 95% confidence interval comparing the intervention group with the usual care group.
We defined adherence as the percentage of prescribed doses taken, which we calculated as the number of doses taken divided by the number of doses prescribed during the observation period times 100%. Adherence was dichotomized at a threshold of 80% because the proportion of pills taken was highly skewed and failed normality assumptions. The 80% cut point has been used as a threshold to assess adherence to medication regimens.38 Analyses were conducted using SAS 9.2 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
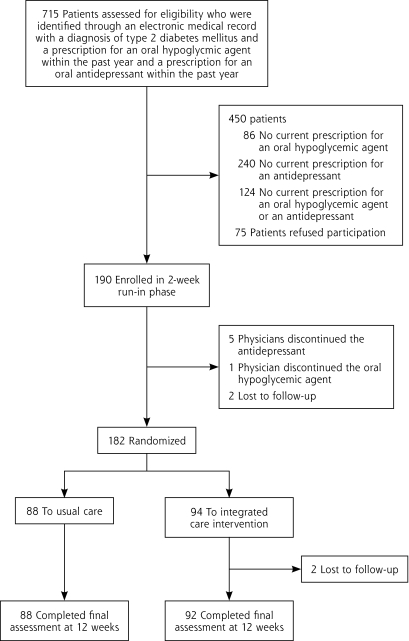
The flow of patients through the trial is depicted in Figure 1. Of 715 patients identified by electronic medical records, 265 were eligible and were approached; 75 refused to participate, and 190 were enrolled (71.7% participation rate). Patients who participated and patients who refused were similar in age, sex, and ethnicity. Consent was followed by a 2-week run-in phase in which adherence to medications was assessed. After randomization at the 2-week meeting, 2 patients in the integrated care intervention were lost to follow-up, but the remaining 180 patients completed the final study visit.
Figure 1.
Study flow diagram.
Sample Characteristics
Baseline characteristics of the 180 patients randomized the intervention or usual care are displayed in Table 1. Baseline characteristics of patients in the integrated care intervention did not differ significantly from those of patients in the usual care group, including adherence rates at baseline measured during the 2-week run-in period.
Table 1.
Baseline Characteristics of Study Population
| Characteristic | Usual Care (n = 88) | Intervention (n = 92) | P Value |
|---|---|---|---|
| Sociodemographic | |||
| Age, mean (SD) y | 57.1 (9.6) | 57.8 (9.4) | .63 |
| African American, n (%) | 48 (54.5) | 54 (58.7) | .30 |
| White, n (%) | 36 (40.9) | 29 (31.5) | |
| Hispanic, n (%) | 3 (3.4) | 4 (4.3) | |
| Other, n (%) | 1 (1.1) | 5 (5.4) | |
| Sex, women n (%) | 58 (65.9) | 64 (69.6) | .64 |
| Less than high school education, n (%) | 15 (17.0) | 14 (15.2) | .84 |
| Type 2 diabetes mellitus | |||
| Years of diabetes, mean (SD) | 12.0 (11.8) | 10.5 (10.2) | .35 |
| HbA1c, mean (SD), % | 7.0 (1.9) | 7.2 (1.8) | .51 |
| Depression | |||
| PHQ-9 score, mean (SD)a | 9.9 (7.2) | 10.6 (7.9) | .54 |
| Medications | |||
| Number of medications, mean (SD) | 10.1 (5.1) | 9.8 (4.5) | .66 |
| ≥ 80% adherent to oral hypoglycemic agent, n (%) | 37 (42.0) | 33 (35.9) | .45 |
| ≥ 80% adherent to antidepressant, n (%) | 34 (39.0) | 28 (30.4) | .27 |
| Functional status (SF-36) scoreb | |||
| Physical function, mean (SD) | 53.6 (31.7) | 50.8 (32.6) | .56 |
| Social function, mean (SD) | 67.7 (39.9) | 76.6 (36.9) | .12 |
| Role physical, mean (SD) | 49.4 (46.7) | 59.5 (46.6) | .15 |
| Role emotional, mean (SD) | 65.9 (46.0) | 67.8 (44.6) | .79 |
| Bodily pain, mean (SD) | 42.3 (31.4) | 50.9 (31.7) | .07 |
| Cognitive status | |||
| MMSE score, mean (SD)c | 28.2 (2.3) | 28.2 (2.3) | .99 |
| Cardiovascular disease risk factors | |||
| Systolic blood pressure, mean (SD), mm Hg | 132.3 (21.4) | 133.9 (20.4) | .62 |
| Diastolic blood pressure, mean (SD), mm Hg | 77.0 (11.4) | 76.6 (11.1) | .85 |
| Body mass index, mean (SD) | 33.8 (8.3) | 34.5 (10.8) | .62 |
| LDL cholesterol,d mean (SD), mg/dL | 96.9 (27.4) | 95.6 (33.4) | .77 |
HbA1c = glycated hemoglobin; LDL = low-density lipoprotein; MMSE = Mini-Mental State Examination; PHQ-9 = 9-item Patient Health Questionnaire; SF-36 = Medical Outcomes Study Short Form.
Note: P Values represent comparisons according to the Fisher exact test and t tests for categorical or continuous data, respectively.
Scored on a range from 0 to 27, where lower scores represent fewer depressive symptoms.
Scored on a range from 0 to 100, where lower scores represent functional impairment.
Scored on a range from 0 to 30, where lower scores represent cognitive impairment.
n = 175 because of missing values.
Adherence Outcomes
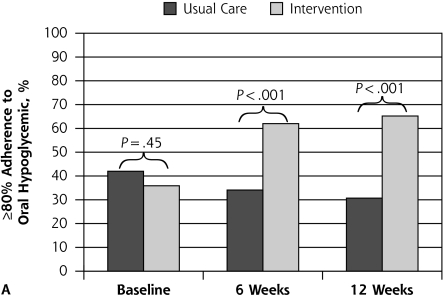
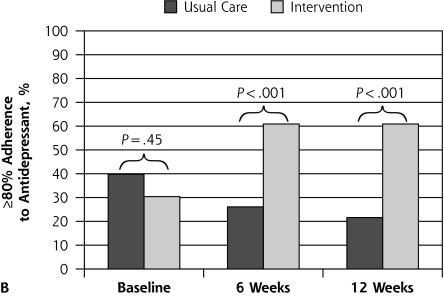
Figure 2 depicts proportions with 80% or greater adherence to oral hypoglycemic agents and antidepressants over time according to treatment assignment. At 6 and 12 weeks, a significant improvement in adherence to oral hypoglycemic agents (P <.001) and antidepressants (P <.001) was seen in the intervention in comparison with usual care.
Figure 2.
Patient outcome of ≥80% adherence to oral hypoglycemic agent and to antidepressant medication.
Note: Assessed with the Medication Event Monitoring System at baseline (preintervention) and at 6 and 12 weeks postintervention, according to treatment assignment.
Clinical Outcomes
Clinical outcomes at 12 weeks are shown in Table 2. Patients randomized to the integrated care intervention were more likely to achieve a HbA1c levels of less than 7% compared with patients in the usual care group at 12 weeks (intervention 60.9% vs usual care 35.7%; P <.001). Patients in the integrated care intervention also had a significantly improved mean change in HbA1c levels from baseline compared with patients in the usual care group at 12 weeks (intervention −0.70 vs usual care 0.50; P <.001). Patients randomized to the integrated care intervention were more likely to achieve remission of depression compared with patients in the usual care group at 12 weeks (intervention 58.7% vs usual care 30.7%; P <.001). Patients in the integrated care intervention also had significantly improved mean change in PHQ-9 scores from baseline compared with patients in the usual care group at 12 weeks (intervention −2.42 vs usual care −0.29; P = .007).
Table 2.
Clinical Outcomes of Glycemic Control and Depression Symptoms in Usual Care and in the Integrated Intervention at 12 Weeks
| Outcomes for Type 2 Diabetes Mellitus | Unadjusted Estimate
|
Estimated Between-Group Odds Ratio or Difference (95% CI) | P Value | |
|---|---|---|---|---|
| Usual Care (n = 88) | Intervention (n = 92) | |||
| Glucose control | ||||
| Achieved HbA1c <7%, n (%) | 25 (35.7) | 67 (60.9) | 8.48 (3.24 to 22.2)a | <.001 |
| HbA1c, change from baseline, mean (SD) | 0.50 (1.11) | −0.70 (1.32) | −1.20 (−1.56 to 0.84)b | <.001 |
| Depression | ||||
| Achieved remission (PHQ-9 <5c), n (%) | 27 (30.7) | 54 (58.7) | 6.15 (2.93 to 12.92)d | <.001 |
| PHQ-9 scorec change from baseline, mean (SD) | −0.29 (5.74) | −2.42 (4.75) | −2.13 (−3.68 to −0.59) | .007 |
HbA1c = glycated hemoglobin; PHQ-9 = 9-item Patient Health Questionnaire; SD = standard deviation.
Estimates, 95% confidence intervals, and P values from the statistical models.
Odds ratio (95% CI) from a logistic regression model.
Mean group difference (95% CI) from a repeated measures linear regression model.
PHQ-9 scored on a range from 0 to 27, where lower scores represent fewer depressive symptoms.
Odds ratio (95% CI) from a repeated measures logistic regression model.
DISCUSSION
The primary goal of this study was to test the effectiveness of an integrated care intervention for patients with type 2 diabetes and depression that focused on adherence compared with usual care for primary care patients. Our principal finding was that primary care patients randomized to the integrated care intervention showed higher rates of adherence to oral hypoglycemics and antidepressants, as well as greater glucose control and fewer depressive symptoms, at the final study visit. Our results show the usefulness of a simple, brief, integrated care management intervention for primary care patients with type 2 diabetes and depression.
Before discussing implications of our findings, the limitations of our study require discussion. First, our results were obtained from patients who receive care at 3 primary care sites, which might not be representative of most primary care practices. The 3 practices, however, were diverse and varied in size, and they were probably similar to other primary care practices in the region. Second, all methods for assessing adherence have limitations. We chose to use MEMS caps as our primary measure of adherence because MEMS caps have a low failure rate38 and may be more sensitive than other adherence measures.39 Any effect of MEMS caps on medication adherence would be experienced equally in both groups. Third, while the 80% threshold for adherence has been assessed in some clinical research (eg, George et al38), the clinical relevance of this threshold has not been tested for many medications. Fourth, patients in the usual care group did not have the same number of in-person contacts as those in the integrated care intervention to control for the effects of attention.
We adopted a comprehensive set of guidelines for the management of depression and type 2 diabetes into an integrated care intervention and applied them in the context of a primary care setting in which most adults receive their medical care. Approximately 90% of all persons with diabetes receive continuous care from primary care physicians.40 No more than 20% of people with diabetes ever see an endocrinologist, and there are not enough endocrinologists to handle the increasing number of people with diabetes.41 The American Diabetes Association has modified its guidelines to recommend routine screening for depression for diabetic patients, particularly those with poor adherence.35 Yet, many primary care patients with type 2 diabetes receive inadequate treatment because of poor adherence to oral hypoglycemic agents. At the same time, the treatment of type 2 diabetes needs to be better integrated with the depression that often co-occurs. Improved management of both type 2 diabetes and depression could have an important public health impact on patient functional status and mortality.42
Our intervention builds on the work of Katon et al,22 because our intervention is brief, does not require a high level of expertise, and is adaptable to community-based primary care settings, where more than one-half of patients seen may be from underrepresented minority groups at high risk for type 2 diabetes and poor outcomes.43–45 A recent review of diabetes self-management interventions noted that an assessment of the feasibility of many interventions is limited by failure to report overall contact time with study patients.12 The total contact time for our intervention was 2 hours (3, 30-minute in-person meetings and 2, 15-minute telephone contacts). Compared with several systematic reviews,10,12,46 we had fewer study visits and substantially less total contact time than most interventions targeting diabetes management.
Our study provides a sustainable solution that can be implemented in primary care or other settings for patients managing multiple medical conditions and varying degrees of complexity in pharmacotherapeutic regimens. Given the low rates of adherence during the first 2 weeks before randomization in our study and the corresponding difficulty patients experience in adhering to physician recommendations for the treatment of depression and type 2 diabetes, interventions that allow for tailoring content and providing tools to match the individualized needs of patients are needed. Ancillary health personnel who are already working in primary care practices could be trained to carry out the intervention. Our results call for greater emphasis within health care systems and policy organizations on the development and promotion of clinical programs to enhance medication adherence, particularly among patients with chronic medical conditions and depression.
Footnotes
Conflicts of interest: authors report none.
Funding support: This work was supported by American Diabetes Association Clinical Research Award 1-09-CR-07. Dr Bogner was supported by NIMH grant MH082799 and MH047447. Dr Morales was supported by a NIMH-mentored Career Development Award (MH073903).
Clinical Trial Registration: Integrating Depression Services Into DM Management, NCT01098253, http://clinicaltrials.gov/show/NCT01098253.
References
- 1.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49(5):837–845 [DOI] [PubMed] [Google Scholar]
- 3.Nouwen A, Winkley K, Twisk J, et al. European Depression in Diabetes (EDID) Research Consortium Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53(12):2480–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications. 2005;19(2):113–122 [DOI] [PubMed] [Google Scholar]
- 5.Simon GE, Katon WJ, Lin EH, et al. Cost-effectiveness of systematic depression treatment among people with diabetes mellitus. Arch Gen Psychiatry. 2007;64(1):65–72 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31 (12):2398–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Korff M, Katon W, Lin EH, et al. Potentially modifiable factors associated with disability among people with diabetes. Psychosom Med. 2005;67(2):233–240 [DOI] [PubMed] [Google Scholar]
- 8.Schram MT, Baan CA, Pouwer F. Depression and quality of life in patients with diabetes: a systematic review from the European depression in diabetes (EDID) research consortium. Curr Diabetes Rev. 2009;5(2):112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koopmans B, Pouwer F, de Bie RA, van Rooij ES, Leusink GL, Pop VJ. Depressive symptoms are associated with physical inactivity in patients with type 2 diabetes. The DIAZOB Primary Care Diabetes study. Fam Pract. 2009;26(3):171–173 [DOI] [PubMed] [Google Scholar]
- 10.Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001;24(10):1821–1833 [DOI] [PubMed] [Google Scholar]
- 11.Williams JW, Jr, Gerrity M, Holsinger T, Dobscha S, Gaynes B, Dietrich A. Systematic review of multifaceted interventions to improve depression care. Gen Hosp Psychiatry. 2007;29(2):91–116 [DOI] [PubMed] [Google Scholar]
- 12.Leeman J. Interventions to improve diabetes self-management: utility and relevance for practice. Diabetes Educ. 2006;32(4):571–583 [DOI] [PubMed] [Google Scholar]
- 13.Olfson M, Marcus SC, Tedeschi M, Wan GJ. Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry. 2006;163(1):101–108 [DOI] [PubMed] [Google Scholar]
- 14.Kodner DL, Spreeuwenberg C. Integrated care: meaning, logic, applications, and implications—a discussion paper. Int J Integr Care. 2002;2:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkrishnan R, Rajagopalan R, Camacho FT, Huston SA, Murray FT, Anderson RT. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: a longitudinal cohort study. Clin Ther. 2003;25(11):2958–2971 [DOI] [PubMed] [Google Scholar]
- 16.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107 [DOI] [PubMed] [Google Scholar]
- 17.Dirmaier J, Watzke B, Koch U, et al. Diabetes in primary care: prospective associations between depression, nonadherence and glycemic control. Psychother Psychosom. 2010;79(3):172–178 [DOI] [PubMed] [Google Scholar]
- 18.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160(21):3278–3285 [DOI] [PubMed] [Google Scholar]
- 19.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042–1049 [DOI] [PubMed] [Google Scholar]
- 20.McKellar JD, Humphreys K, Piette JD. Depression increases diabetes symptoms by complicating patients’ self-care adherence. Diabetes Educ. 2004;30(3):485–492 [DOI] [PubMed] [Google Scholar]
- 21.Petrak F, Herpertz S. Treatment of depression in diabetes: an update. Curr Opin Psychiatry. 2009;22(2):211–217 [DOI] [PubMed] [Google Scholar]
- 22.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angst J. Clinical course of affective disorders. In: Helgason T, Daly R, eds. Depression Illness: Prediction of Course and Outcome. Berlin, Germany: Springer-Verlag; 1988:1–47 [Google Scholar]
- 24.Kahn R, Alperin P, Eddy D, et al. Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet. 2010;375(9723):1365–1374 [DOI] [PubMed] [Google Scholar]
- 25.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391 [PubMed] [Google Scholar]
- 26.Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African-American, Hispanic, and white primary care patients. Med Care. April 2003;41(4):479–489 [DOI] [PubMed] [Google Scholar]
- 27.Bogner HR, de Vries HF: Integration of depression and hypertension treatment: a pilot, randomized controlled trial. Ann Fam Med. 2008;6: (4)295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogner HR, de Vries HF. Integrating type 2 diabetes mellitus and depression treatment among African Americans: a randomized controlled pilot trial. Diabetes Educ. 2010;36(2):284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mundt JC, Clarke GN, Burroughs D, Brenneman DO, Griest JH. Effectiveness of antidepressant pharmacotherapy: the impact of medication compliance and patient education. Depress Anxiety. 2001;13(1):1–10 [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198 [DOI] [PubMed] [Google Scholar]
- 31.American Heart Association Blood pressure testing and assessment. http://www.americanheart.org Accessed Dec 14, 2010
- 32.Carnethon MR, Kinder LS, Fair JM, Stafford RS, Fortmann SP. Symptoms of depression as a risk factor for incident diabetes: findings from the National Health and Nutrition Examination Epidemiologic Follow-up Study, 1971–1992. Am J Epidemiol. 2003;158(5):416–423 [DOI] [PubMed] [Google Scholar]
- 33.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143. [PubMed] [Google Scholar]
- 34.Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26(7):724–735 [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association Clinical practice recommendations. Diabetes Care. 2010;33(Suppl 1):S1–S100 [PubMed] [Google Scholar]
- 36.Moridani MY, Verjee Z, Allen LC. Analytical evaluation of hemoglobin A(1c) dual kit assay on Bio-Rad Variant II: an automated HPLC hemoglobin analyzer for the management of diabetic patients. Clin Biochem. 2003;36(4):317–320 [DOI] [PubMed] [Google Scholar]
- 37.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George CF, Peveler RC, Heiliger S, Thompson C. Compliance with tricyclic antidepressants: the value of four different methods of assessment. Br J Clin Pharmacol. 2000;50(2):166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):1074–1090, discussion 1073. [DOI] [PubMed] [Google Scholar]
- 40.Smith DM. Toward common ground. Diabetes Care. 1997;20(4): 467–468 [DOI] [PubMed] [Google Scholar]
- 41.Saudek CD. The role of primary care professionals in managing diabetes. Clin Diabetes. 2002;20(2):65–66 [Google Scholar]
- 42.Bogner HR, Morales KH, Post EP, Bruce ML. Diabetes, depression, and death: a randomized controlled trial of a depression treatment program for older adults based in primary care (PROSPECT). Diabetes Care. 2007;30(12):3005–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wisdom K, Fryzek JP, Havstad SL, Anderson RM, Dreiling MC, Tilley BC. Comparison of laboratory test frequency and test results between African-Americans and Caucasians with diabetes: opportunity for improvement. Findings from a large urban health maintenance organization. Diabetes Care. 1997;20(6):971–977 [DOI] [PubMed] [Google Scholar]
- 44.Harris MI. Racial and ethnic differences in health care access and health outcomes for adults with type 2 diabetes. Diabetes Care. 2001;24(3):454–459 [DOI] [PubMed] [Google Scholar]
- 45.Kirk JK, D’Agostino RB, Jr, Bell RA, et al. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care. 2006;29(9):2130–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harkness E, Macdonald W, Valderas J, Coventry P, Gask L, Bower P. Identifying psychosocial interventions that improve both physical and mental health in patients with diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(4):926–930 [DOI] [PMC free article] [PubMed] [Google Scholar]