Abstract
The activator protein–1 (AP-1) transcription factor, comprising Jun and Fos family proteins, distinctly regulates various cellular processes, including those involved in inflammation. FOS like antigen 1 (Fra-1), a member of the Fos family, dimerizes with members of the Jun family and regulates gene expression in a context-dependent manner. Although respiratory toxicants are known to stimulate the expression of Fra-1 in the lung, whether Fra-1 promotes or decreases susceptibility to the development and progression of toxicant-induced lung disease in vivo is not well established. To determine the role of Fra-1 in LPS-induced acute lung injury and mortality, we administered LPS either intraperitoneally or intratracheally to Fra-1–sufficient (Fra-1+/+) and Fra-1–deficient (Fra-1Δ/Δ) mice. LPS-induced mortality, lung injury, inflammation, cytokine measurements, and AP-1 and NF-κB activities were then assessed in these mice. Fra-1Δ/Δ mice showed a greater resistance to LPS-induced mortality than did their Fra-1+/+counterparts. Consistent with this result, LPS-induced lung injury and inflammatory responses were markedly lower in Fra-1Δ/Δ mice than in Fra-1+/+ mice. Compared with Fra-1+/+ mice, Fra-1Δ/Δ mice showed a reduced influx of neutrophils into the lungs, accompanied by a decreased expression of proinflammatory cytokines in response to treatment with LPS. The decreased inflammatory responses in Fra-1Δ/Δ mice coincided with diminished and increased levels of NF-κB and c-Jun/AP-1 binding, respectively. These results demonstrate that Fra-1/AP-1 plays a key role in promoting LPS-induced injury and mortality in mice, and they suggest that targeting (i.e., inhibiting) this transcription factor may be a useful approach to dampening the adverse effects of exposure to endotoxins.
Keywords: acute lung injury, inflammation, Fra-1, cytokines, host defense
Clinical Relevance
The results of the present study suggest that the Fra-1/activator protein–1 (AP-1) transcription factor contributes to LPS-induced lung injury and mortality in mice. Fra-1 mediates LPS-induced effects by regulating proinflammatory cytokine gene expression through the modulation of c-Jun and NF-κB signaling. This study identifies Fra-1 as a potentially useful target for inhibiting lung injury and inflammation in patients with acute lung injury and adult respiratory distress syndrome, and for minimizing the effects of exposure to endotoxins in clinical settings.
Acute lung injury (ALI) and its severe form, adult respiratory distress syndrome (ARDS), are devastating clinical entities, caused by bacterial products and other injurious agents. These pathological conditions afflict over a million patients each year and cause major public health problems throughout the world. Approximately 35% of patients with severe ALI/ARDS die in the hospital during treatment, and those who survive manifest persistent health problems (1). Although various injurious experimental models were used to define the mechanisms underlying ALI/ARDS, only very few potentially effective therapies are available to treat these clinical syndromes. Thus, furthering our understanding of the mechanisms underlying ALI is critical to devising better and more effective therapies for these conditions. Evidence obtained from experimental models demonstrated that LPS binds to the Toll-like receptor 4 (TLR-4) and induces a signaling cascade that leads to the activation of various effector pathways. In particular, LPS induces NF-κB and activator protein–1 (AP-1), resulting in the increased production of various inflammatory mediators during lung injury and inflammation, with ensuing morbidity and mortality (2, 3). Although the role of NF-κB in mediating ALI was well established via genetic and pharmacological approaches (4, 5), the exact role of AP-1 in regulating LPS-induced ALI/ARDS remains largely unknown.
AP-1 is a dimeric transcription factor mainly composed of Jun (c-Jun, Jun-B, and Jun-D), Fos (c-Fos, Fos-B, Fra-1, and Fra-2), and activating transcription factor (ATF) family members. AP-1 binds to the 12-O-tetradecanoylphorbol-13-acetate (TPA) response element (the TRE or AP-1 site) in the promoter regions of genes encoding several cytoprotective enzymes, cytokines, and growth factors, and distinctly regulates their transcription in a context-dependent manner (6). AP-1 plays a significant role in mediating inflammatory responses by regulating the production of a number of cytokines and chemokines (7). For example, mice lacking c-Fos exhibit an enhanced production of inflammatory cytokines in response to LPS (8) and enhanced susceptibility to dextran sulfate–induced colitis (9), in part as a result of the activation of NF-κB. The conditional deletion of c-Jun and Jun-B in the epidermis of adult mice leads to psoriasis, accompanied by the enhanced expression of inflammatory mediators (10), suggesting that Jun proteins control the development of skin disorders associated with tissue inflammation.
In addition to mitogenic stimuli, a number of toxicants stimulate the expression of Fra-1 in various cell types of several organs, including the lung (11). In contrast to the early activation of c-Jun and c-Fos, the delayed induction of Fra-1 by toxic and oxidant stimuli is postulated to play a critical role in modulating the persistent transcriptional response initiated by AP-1 (11–13). However, the exact role of Fra-1 in mediating ALI induced by injurious insults is not well defined in vivo. Here, using a murine model with the conditional deletion of Fra-1, we provide experimental evidence that Fra-1/AP-1 plays an obligatory role in LPS-induced lung inflammatory responses and mortality, and we suggest that this transcription factor may be a promising therapeutic target for ALI/ARDS.
Some of our results were previously reported in the form of an abstract at the international conference of the American Thoracic Society in May 2010 (14).
Materials and Methods
Mice
Mice bearing “floxed” alleles of Fra-1 (Fra-1F/F) on a C57BL/6J × 129 background were obtained from Erwin Wagner (Spanish National Cancer Research Center, Madrid, Spain). The conventional deletion of Fra-1 leads to embryonic lethality, attributable to the requirement of Fra-1–dependent gene expression for the development of extraembryonic tissue (15). Fra-1 conditional knockout mice (Fra-1Δ/Δ) were produced by crossing Fra-1F/F mice with mesenchyme homeobox 2 (Meox2)-Cre mice (Jackson Laboratory, Bar Harbor, Maine) as previously described (16). Meox2 Cre mice express Cre recombinase, driven by the 5′-flanking region of Meox2 and mainly restricted to embryonic tissue, and not extraembryonic tissue (17). Their wild-type littermates did not express Meox2-Cre (designated Fra-1+/+), and were used as controls. Mice were genotyped for the presence of floxed or deleted Fra-1 alleles as well as Meox2-Cre alleles (16). All experimental animal protocols were approved by Johns Hopkins University (Baltimore, MD).
Treatment with LPS
Fra-1+/+ and Fra-1Δ/Δ (8-week-old) mice received sterile PBS (vehicle) or LPS (catalogue number L4005; Sigma, St. Louis, MO) by either intraperitoneal injection or intratracheal instillation. Mice were killed at the indicated times after challenge with LPS. For survival studies, mice were challenged with LPS (40 or 60 mg/kg body weight), intraperitoneal and monitored for 5 days, and their times of death were recorded.
Assessment of Lung Injury and Inflammation
Bronchoalveolar lavage (BAL) fluid was collected from the right lung by an instillation with 1.5 ml (0.75 ml, twice) of sterile PBS. The left lung was used for RNA extraction or fixed in formalin for histologic examination. To measure lung inflammation, differential cell counts in BAL fluid were evaluated with the Diff-Quik stain set (Dade-Behring, Newark, DE). Lung injury (i.e., alveolar permeability) was evaluated by measuring protein concentrations in the BAL fluid with a BCA protein assay kit (Pierce Thermoscientific, Rockford, IL).
Real-Time RT-PCR Analyses
Total RNA was isolated and real-time RT-PCR reactions were performed, using TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA).
Cytokine Measurements
Cytokine levels in cell-free BAL samples were quantitated using a Bio-Plex Mouse Cytokine 23-Plex Assay Kit (Bio-Rad Laboratories, Hercules, CA). Levels of active TGF-β1 and macrophage inflammatory protein-2 alpha (MIP-2α) were measured using ELISA kits (eBioscience, Inc., San Diego, CA, and R&D Systems, Minneapolis, MN, respectively).
Culture of Peritoneal Macrophages
Peritoneal macrophages, as elicited by thioglycollate, were isolated and seeded on plates in RPMI-1640 medium supplemented with 10% FBS. Adherent cells were cultured overnight before treatment with LPS.
NF-κB and c-Jun Binding Assays
TransAM AP-1 and TransAM NF-κB kits (Active Motif, Carlsbad, CA) were used to quantify the binding of c-Jun to the AP-1 site and p65 to the NF-κB site, according to the manufacturer's instructions.
Lung Tissue Myeloperoxidase Activity
Lung tissue was homogenized, sonicated, and centrifuged, and the supernatant was mixed 1/10 (vol/vol) with assay buffer (0.167 mg/ml O-dianisidine hydrochloride and 0.0006% H2O2). Changes in absorbance were measured at 460 nm, and the activity of myeloperoxidase (MPO) was calculated as the change in absorbance over time, and was normalized according to lung weight.
Statistical Analysis
Data were expressed as means ± SD. Student two-tailed t tests were used to determine significant differences between various experimental groups. P ≤ 0.05 was considered statistically significant.
Results
Fra-1 Deficiency Confers Protection from LPS-Induced Death In Vivo
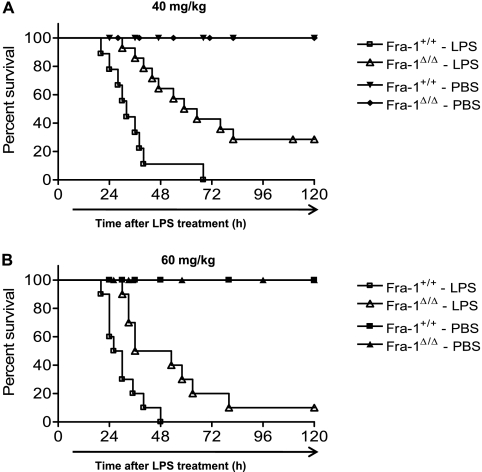
To examine the role of Fra-1 in LPS-induced mortality, age-matched Fra-1+/+ and Fra-1Δ/Δ mice were challenged with two lethal doses of LPS or PBS (vehicle). Mice were injected intraperitoneally with 40 mg/kg or 60 mg/kg LPS or PBS, and their survival was monitored for 5 days. As shown in Figure 1, Fra-1Δ/Δ mice exhibited a significantly prolonged survival compared with their wild-type littermate controls at both doses of LPS. An approximately two-fold increase occurred in the survival time of Fra-1Δ/Δ mice treated with 40 mg/kg LPS. The Fra-1Δ/Δ mice demonstrated a median survival time of 62 hours, compared with 32 hours for the Fra-1+/+ mice (Figure 1A). Treatment with the higher dose of LPS also produced a significant difference in median survival time (i.e., 44.5 hours for Fra-1Δ/Δ mice compared to 28 hours for their wild-type counterparts) (Figure 1B). These results show that the deletion of Fra-1 leads to greater protection from LPS-induced mortality in vivo.
Figure 1.
The deletion of Fra-1 confers protection against LPS-induced mortality. (A and B) Kaplan-Meier survival curves of Fra-1–sufficient (Fra-1+/+) and Fra-1–deficient (Fra-1Δ/Δ) mice injected with intraperitoneal LPS (n = 10 for each genotype) at doses of 40 mg/kg (A) and 60 mg/kg (B) or vehicle PBS (n = 6 for each genotype). Survival of Fra-1Δ/Δ mice was significantly enhanced, compared with that of Fra-1+/+ mice, at both concentrations of LPS. Differences in mortality were assessed according to log-rank test (P < 0.05).
Fra-1 Deficiency Attenuates Lung Injury and Inflammation Induced by Systemic LPS
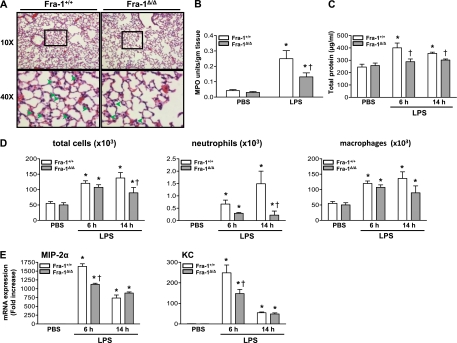
We then asked whether Fra-1 promotes LPS-induced death by enhancing lung injury and inflammation in vivo. To answer this question, Fra-1+/+ and Fra-1Δ/Δ mice were injected intraperitoneally with 40 mg/kg LPS and killed at 6 or 14 hours after the administration of LPS. Lungs were fixed or lavaged for the collection of the BAL fluid to measure lung injury and inflammation. Histopathological analysis of lungs harvested from mice treated with LPS for 14 hours revealed a decrease in the number of neutrophils recruited to the lung tissue (interstitium) in Fra-1Δ/Δ mice compared with Fra-1+/+ mice (Figure 2A). To quantify the neutrophils recruited into lung tissue, we measured the activity of MPO in the lungs of Fra-1Δ/Δ and Fra-1+/+ mice treated with vehicle or LPS (Figure 2B). In agreement with the histological analysis, we found significantly decreased levels of MPO activity in the lungs obtained from Fra-1Δ/Δ mice treated with LPS, compared with levels of MPO activity in the lungs of their wild-type counterparts. Likewise, protein leakage into the BAL fluid of Fra-1Δ/Δ mice treated with LPS for 6 or 14 hours was significantly lower than in their Fra-1+/+ counterparts (Figure 2C, open versus solid bars).
Figure 2.
Effect of systemic LPS on lung injury and inflammation. Fra-1+/+ and Fra-1Δ/Δ mice (n = 6 for each genotype) were treated with intraperitoneal LPS (40 mg/kg body weight) or vehicle control (n = 4 for each genotype), and lung injury was assessed. (A) Histopathological analysis of lung tissue obtained from Fra-1+/+ and Fra-1Δ/Δ mice treated with intraperitoneal LPS for 14 hours. Representative hematoxylin-and-eosin–stained images of lungs are shown at ×10 magnification (above) and ×40 magnification (below, from areas framed above). Arrows indicate neutrophils. (B) Quantitation of myeloperoxidase (MPO) activity in lung tissue obtained from Fra-1+/+ and Fra-1Δ/Δ mice treated with intraperitoneal LPS for 14 hours. (C) Total protein in the bronchoalveolar lavage (BAL) fluid obtained from Fra-1+/+ and Fra-1Δ/Δ mice treated with vehicle or LPS. (D) Total and inflammatory cells in the BAL fluid from Fra-1+/+ and Fra-1Δ/Δ mice treated with vehicle or LPS. (E) Quantitative RT-PCR analysis of macrophage inflammatory protein-2 alpha (MIP-2α) and keratinocyte-derived chemokine (KC) expression in lung tissue after treatment with LPS. *P < 0.05, PBS versus LPS. †P < 0.05, Fra-1+/+ mice versus Fra-1Δ/Δ mice.
A differential cell count analysis revealed significant increases in the number of total cells, neutrophils, and macrophages in both Fra-1+/+ and Fra-1Δ/Δ mice at 6 and 14 hours after treatment with LPS. However, a significantly lower number of neutrophils was evident in the BAL fluid of Fra-1Δ/Δ mice treated with LPS for 14 hours than in the BAL fluid of their Fra-1+/+ counterparts. No significant difference was evident in total cell numbers between the two genotypes at 6 hours after LPS treatment (Figure 2D). To determine whether any change in the expression of inflammatory cytokines had occurred, RNA was extracted from the lungs of mice treated with vehicle or LPS, and the expression of proinflammatory cytokines was analyzed using quantitative RT-PCR. We observed elevated expression levels of several inflammatory cytokines, including MIP-2α and keratinocyte-derived chemokine (KC), at both time points. However, the expression levels of these two cytokines were markedly lower at 6 hours after treatment in Fra-1Δ/Δ mice. The expression of MIP-2α and KC had decreased by 14 hours in both Fra-1+/+ and Fra-1Δ/Δ mice. No significant difference was evident in expression levels between the two genotypes at this time point (Figure 2E).
Deletion of Fra-1 Diminishes Intratracheal LPS-Induced Acute Lung Injury
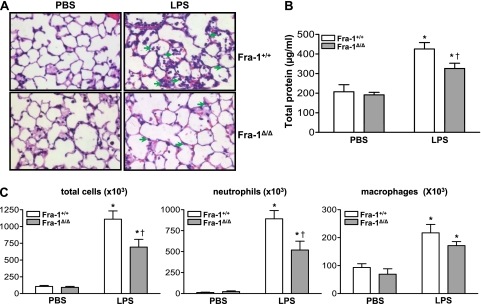
We next evaluated the effects of Fra-1 deficiency on lung injury and inflammation in response to the instillation of LPS or PBS Both Fra-1+/+ and Fra-1Δ/Δ mice were instilled with 10 μg LPS or PBS and killed after 24 hours. Lungs were harvested for histologic and inflammatory cell analyses. Histopathological evaluations revealed a decreased number of infiltrating neutrophils in the lungs of Fra-1Δ/Δ mice treated with LPS compared with their Fra-1+/+ counterparts (Figure 3A). A significantly lower total protein concentration was found in the BAL fluid obtained from Fra-1Δ/Δ mice treated with LPS than in the BAL fluid obtained from LPS-treated Fra-1+/+ mice (Figure 3B). LPS caused a marked increase in the number of neutrophils in the BAL fluid of both wild-type and knockout mice. However, consistent with the histologic examination of lung tissue and BAL protein analysis, significantly lower numbers of total cells and neutrophils were recruited to the lungs of Fra-1Δ/Δ mice than to the lungs of Fra-1+/+ mice (Figure 3C).
Figure 3.
Effect of Fra-1 deficiency on intratracheal LPS-induced acute lung injury (ALI). Fra-1+/+ and Fra-1Δ/Δ mice were instilled with PBS (n = 4 for each genotype) or LPS (10 μg/ mouse) (n = 6 for each genotype) via the intratracheal route, and killed after 24 hours. Lung injury and inflammation were assessed. (A) Hematoxylin-and-eosin–stained images of lung histopathology in Fra-1+/+ and Fra-1Δ/Δ mice. Arrows indicate presence of neutrophils in the alveolar spaces. (B) Total protein and (C) total and inflammatory cells in the BAL fluid of Fra-1+/+ and Fra-1Δ/Δ mice treated with PBS or LPS for 24 hours. *P < 0.05, PBS versus LPS. †P < 0.05, Fra-1+/+ mice versus Fra-1Δ/Δ mice.
Disruption of Fra-1 Diminishes the Expression of LPS-Induced Inflammatory Cytokines
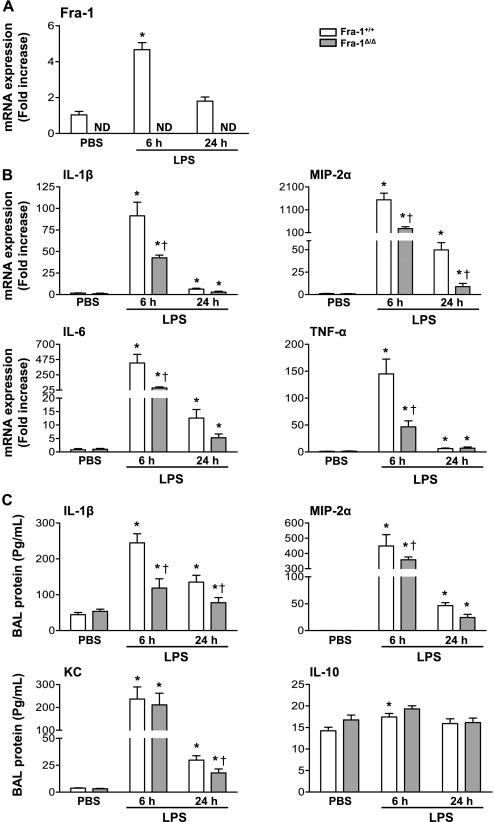
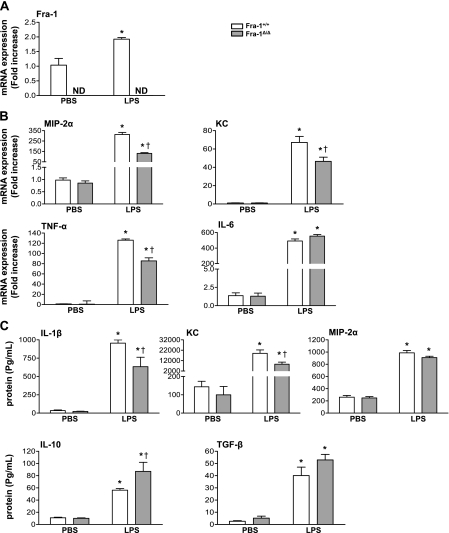
We then asked whether the disruption of Fra-1 would alter the expression of cytokines in the lung in response to intratracheal LPS. RNA was extracted from lungs obtained from Fra-1+/+ and Fra-1Δ/Δ mice at 6 hours or 24 hours following intratracheal instillation of LPS, and the expression levels of Fra-1 and proinflammatory cytokines were quantitated by quantitative RT-PCR (Figure 4). The expression of Fra-1 in wild-type mice was significantly induced by LPS at 6 hours, and it returned to near-basal level at 24 hours (Figure 4A), whereas its expression, as expected, was not detected in the lungs of Fra-1Δ/Δ mice with or without treatment with LPS (Figure 4A). LPS significantly increased the expression levels of IL-1β, IL-6, MIP-2α, and TNF-α in both wild-type and knockout mice (Figure 4B). However, the Fra-1Δ/Δ mice showed a significantly reduced expression of these cytokines at 6 hours after treatment with LPS. In the case of MIP-2α, the decreased expression in Fra-1Δ/Δ mice was evident as late as 24 hours after treatment with LPS. A reduction in the expression of IL-1β and IL-6 was also observed in samples obtained from Fra-1Δ/Δ mice after 24 hours, but this decrease was not statistically significant (Figure 4B). We also determined the concentrations of these cytokines in cell-free BAL fluid by ELISA. This analysis revealed significantly lower levels of LPS-induced IL-1β, MIP-2α, and KC expression in Fra-1Δ/Δ mice than in Fra-1+/+ mice (Figure 4C). We also analyzed the expression levels of IL-10, and TGF-β to determine whether Fra-1 deficiency leads to increased levels of anti-inflammatory cytokine expression (Figure 4C). Although we found slightly elevated levels of IL-10 in the BAL of Fra-1Δ/Δ mice treated with vehicle or LPS for 6 hours, compared with Fra-1+/+ mice, these levels were not statistically significantly different between the two genotypes (Figure 4C). We found very low or undetectable levels of active TGF-β in the BAL of both vehicle-treated mice and Fra-1+/+ and Fra-1Δ/Δ mice treated for 6 hours or 24 hours with LPS under our experimental conditions (data not shown). These results suggest that Fra-1 regulates LPS-induced inflammatory responses in the lung by modulating mainly proinflammatory cytokine gene expression.
Figure 4.
Expression of inflammatory cytokines in lungs of Fra-1+/+ and Fra-1Δ/Δ mice after instillation of LPS. Fra-1+/+ and Fra-1Δ/Δ mice were instilled with PBS (n = 4 for each genotype) or LPS (10 μg/ mouse) (n = 6 for each genotype), and killed after 6 hours or 24 hours. (A) Quantitative RT-PCR analysis of Fra-1 expression in the lung after administration of LPS. (B) Quantitative RT-PCR analysis of LPS-induced inflammatory cytokines in the lung. (C) Levels of cytokines in cell-free BAL fluid, as analyzed by Bioplex assay and ELISA (MIP-2α). *P < 0.05, PBS versus LPS. †P < 0.05, Fra-1+/+ mice versus Fra-1Δ/Δ mice.
To confirm the role of Fra-1 in the regulation of LPS-mediated inflammatory responses, we examined the effects of LPS on the induction of proinflammatory cytokines in Fra-1+/+ and Fra-1Δ/Δ macrophages in vitro. Peritoneal macrophages isolated from Fra-1+/+ and Fra-1Δ/Δ mice were treated with LPS for 3 hours, after which the induction of Fra-1 as well as proinflammatory genes was evaluated by quantitative RT-PCR (Figure 5). As anticipated, the expression of Fra-1 in Fra-1+/+ macrophages was significantly induced by LPS, but was not detectable in Fra-1Δ/Δ cells (Figure 5A). We next determined whether Fra-1 deficiency alters the expression levels of inflammatory cytokines in macrophages, thereby decreasing susceptibility to LPS. LPS markedly stimulated the expression of MIP-2α, KC, TNF-α, and IL-6 in both wild-type and knockout cells, but the levels of MIP-2α, KC, and TNF-α were markedly lower in Fra-1Δ/Δ cells than in their wild-type counterparts (Figure 5B). We obtained similar results in primary cultured macrophages obtained from bone marrow (data not shown).
Figure 5.
The deletion of Fra-1 alters the LPS-induced expression of inflammatory cytokines in peritoneal macrophages in vitro. Peritoneal macrophages collected from Fra-1+/+ and Fra-1Δ/Δ mice were treated with PBS (n = 5 for each genotype) or LPS (100 ng/mL) (n = 5 for each genotype). (A) Quantitative RT-PCR analysis of Fra-1 expression 3 hours after treatment with LPS. (B) Quantitative RT-PCR analysis of inflammatory cytokines, 3 hours after treatment with LPS. (C) Quantitation of cytokine levels in culture supernatants treated with PBS or LPS for 24 hours, according to Bioplex assay and ELISA (TGF-β and MIP-2α). *P < 0.05, PBS versus LPS. †P < 0.05, Fra-1+/+ cells versus Fra-1Δ/Δ cells.
We next analyzed the protein levels of these cytokines in culture supernatants obtained from macrophages treated with LPS for 24 hours. Fra-1+/+ cells secreted significantly higher levels of IL-1β and KC, compared with their Fra-1Δ/Δ counterparts (Figure 5C). On the other hand, higher levels of IL-10, an anti-inflammatory cytokine, were detected in LPS-treated Fra-1Δ/Δ macrophages than in Fra-1+/+ cells. The levels of TGF-β were also higher (Figure 5C, solid bars), though not to a statistically significant extent, in the culture medium obtained from Fra-1Δ/Δ cells treated with LPS compared with their wild-type counterparts (Figure 5C, open bars). These results further corroborate the findings observed in the lung tissue, and support our contention that the Fra-1/AP-1 pathway differentially modulates the LPS-stimulated expression of pro-inflammatory and anti-inflammatory cytokines.
Fra-1 Distinctly Regulates LPS-Induced c-Jun/AP-1 and NF-κB Binding In Vitro and In Vivo
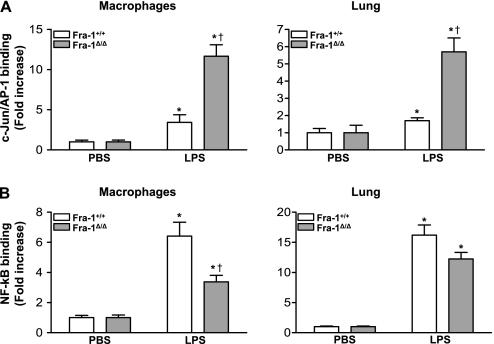
To identify the factors contributing to the increased resistance to LPS-induced lung inflammation in the absence of Fra-1, we investigated the binding of c-Jun, a major component of AP-1 and a dimeric partner of Fra-1. AP-1 complexes composed of Fra-1/Jun proteins bind to AP-1 sites in the promoters of various genes, and regulate their transcription in a context-dependent manner (18). To determine whether the loss of Fra-1 leads to an altered binding of c-Jun to DNA after treatment with LPS, we isolated macrophages from the peritonea of Fra-1+/+ and Fra-1Δ/Δ mice and treated them with LPS for 1 hour. c-Jun binding to the AP-1 site was analyzed using a DNA binding ELISA kit. As anticipated, LPS induced c-Jun binding to the AP-1 site in both Fra-1+/+ and Fra-1Δ/Δ macrophages, compared with their respective vehicle-treated control samples (Figure 6A, left). However, we found a 3.5-fold increase in the levels of c-Jun binding in LPS-treated Fra-1Δ/Δ macrophages compared with their Fra-1+/+ counterparts. To verify this result in vivo, mice were treated intratracheally with 10 μg of LPS for 1 hour. Nuclear extracts were then prepared from lung tissue, and c-Jun binding was analyzed (Figure 6A, right). A stimulation of c-Jun binding was evident in the lung tissue of both Fra-1+/+ and Fra-1Δ/Δ mice after the instillation of LPS. However, the lungs of Fra-1Δ/Δ mice displayed significantly higher levels of c-Jun binding.
Figure 6.
Analysis of LPS-induced c-Jun/activator protein–1 (AP-1) and NF-κB binding activity in vitro and in vivo. Peritoneal macrophages were treated with vehicle (n = 4 for each genotype) or LPS (100 ng/mL) (n = 5 for each genotype) for 1 hour. Nuclear protein extracts were prepared and used to measure binding activity. Lung tissue was harvested 1 hour after the intratracheal instillation of LPS (10 μg/mouse), and nuclear extracts were prepared and used for the binding assays. (A) Quantitation of c-Jun/AP-1 binding in peritoneal macrophages (left) and lung tissue (right) after treatment with LPS. c-Jun/AP-1 binding was measured with a TransAM AP-1 ELISA kit. (B) Quantitation of NF-κB activation in peritoneal macrophages (left) and lung tissue (right) after treatment with LPS. NF-κB binding was measured with a TransAM NF-κB p65 ELISA kit. *P < 0.05, PBS versus LPS. †P < 0.05, Fra-1+/+ genotype versus Fra-1Δ/Δ genotype. Data represent the average of five independent samples.
NF-κB plays a vital role in LPS-induced inflammation by regulating the expression of various inflammatory genes in the lungs. We therefore asked whether Fra-1 modulates the activity of NF-κB under our experimental conditions. For this purpose, we used a DNA binding ELISA kit to analyze NF-κB–DNA binding in nuclear extracts isolated from peritoneal macrophages (Figure 6B, left) and lung tissue (Figure 6B, right), as detailed in Figure 6A. As expected, LPS caused a significant increase in NF-κB binding in both Fra-1+/+ and Fra-1Δ/Δ macrophages, but the binding was significantly lower in Fra-1 mutant cells than in wild-type cells (Figure 6B, left). A similar decrease in NF-κB binding was also evident in lung tissue from Fra-1Δ/Δ mice compared with Fra-1+/+ mice, but this difference was not statistically significant (Figure 6B, right).
Discussion
This study demonstrated an important role for Fra-1 in potentiating LPS-induced ALI and inflammatory responses in vivo. The genetic disruption of Fra-1 markedly decreased the susceptibility of Fra-1–deficient mice to LPS-induced ALI and inflammation, as well as mortality (Figures 1–3). The decreased susceptibility of Fra-1–deficient mice to LPS coincided with a significantly reduced level of neutrophilic inflammation and lung injury (i.e., alveolar permeability). These differences were more apparent after the administration of LPS directly into the lung via the intratracheal route. Consistent with the decreased neutrophilic inflammation were the diminished levels of inflammatory cytokine expression in the BAL fluid and lung tissue that we observed in Fra-1 deficient mice in response to treatment with LPS (Figure 4). Collectively, these results indicate that Fra-1 is an important mediator of LPS-induced ALI and inflammation and of enhanced mortality in vivo, and this transcription factor exerts its effects through modulating the production of inflammatory cytokines.
Inflammatory cytokines play fundamental roles in the pathogenesis of ALI (19, 20). For example, MIP-2α plays an important role in attracting neutrophils into the lung during the inflammatory response, whereas KC chemokine (C-X-C) ligand-1 (Cxcl1) regulates chemotaxis and the activation of neutrophils (19, 20). The inflammatory cytokines (IL-1β, IL-6, and TNF-α) are known to promote inflammation in the lung and other tissues (19, 20). In our study, we observed diminished levels of MIP-2α, IL-1β, IL-6, and TNF-α production in the lungs of Fra-1Δ/Δ mice in response to a nonlethal injection or instillation of LPS (Figures 2 and 3). Moreover, the levels of MIP-2α remained persistently lower even at 24 hours post-treatment with LPS. Thus, the reduced numbers of neutrophils observed in BAL fluids obtained from mice lacking Fra-1 could reflect decreased levels of the LPS-induced expression of MIP-2α and KC. In addition, Fra-1Δ/Δ mice demonstrated elevated levels of anti-inflammatory cytokines in the lung tissue (IL-10; Figure 4C) and macrophages (IL-10 and TGF-β; Figure 5C) in response to LPS, compared with their wild-type counterparts. Thus, we propose that the Fra-1 transcription factor predominantly contributes to LPS-induced ALI by enhancing the expression of pro-inflammatory cytokines, and also by attenuating levels of anti-inflammatory cytokines.
AP-1 acts as one of the key downstream effectors of LPS-activated, TLR-4–mediated signaling (7, 21). AP-1 mediates the LPS-induced inflammatory response by inducing the expression of various cytokines and chemokines that are critical to the recruitment of leukocytes and immune cells, and AP-1 also induces their retention in target tissues (7, 21). c-Fos, a member of the Fos family, negatively regulates the LPS-induced expression of proinflammatory cytokines. For example, the increased susceptibility of Fos-deficient mice to experimental colitis is associated with elevated levels of proinflammatory cytokines (8, 9, 22, 23), and c-Fos–null macrophages exhibit higher levels of IL-12 in response to LPS than do their wild-type counterparts (22). c-Fos–deficient mice displayed an enhanced level of LPS-induced systemic inflammation and severe hypothermia, mainly attributable to enhanced levels of TNF-α in serum and NF-κB activity in macrophages, suggesting that c-Fos negatively regulates LPS-induced inflammation in vivo (8). The combined deletion of c-Jun and Jun-B in the epidermis of adult mice causes psoriasis, accompanied by increased levels of cytokines and chemokines, suggesting a protective role for Jun proteins in inflammatory skin disorders (10, 24). Jun-B positively regulates the expression of IL-4, and the deletion of Jun-B in Th2 cells causes a deregulation of Th2-specific cytokine expression (25, 26). Reduced levels of Jun-B expression were also evident in the skin of patients with lupus, and the conditional deletion of this transcription factor in epidermal cells results in a lupus phenotype and skin ulcerations, accompanied by increased levels of cytokines such as IL-6 (27, 28). Our results suggest that the Fra-1–dependent transcriptional response plays a key role in regulating the expression of these cytokines in response to treatment with LPS (Figure 4). Because Jun proteins negatively regulate both inflammatory and immune responses, and because the overexpression of Fra-1 dampens the activity of c-Jun/AP-1 (29–31), the attenuated lung inflammation seen in Fra-1Δ/Δ mice in response to LPS treatment is likely attributable to increased levels of Jun and c-Fos proteins binding to the AP-1 site. Consistent with this mechanism, our DNA-binding ELISA analysis revealed higher levels of c-Jun binding in both the lungs and cultured macrophages of Fra-1Δ/Δ mice treated with LPS than in their wild-type counterparts (Figure 6A). Alternatively, the attenuated lung inflammation in Fra-1Δ/Δ mice after exposure to LPS may be attributable to the formation of c-Jun–based AP-1 complexes composed of ATF and other Fos family members. This possibility remains to be investigated under our experimental conditions.
The results of our lethal and nonlethal LPS challenges strongly support the concept that Fra-1 is an important factor in the increased production of proinflammatory cytokines in the lung as well as in freshly isolated macrophages. Paradoxically, other studies showed that Fra-1 both positively and negatively regulates the production of proinflammatory cytokines in cultured cells. For example, Fra-1 binds to the IL-6 promoter, thereby increasing its production in macrophages (32). On the other hand, Fra-1 exerts an inhibitory effect on the IL-1β–induced transcription of IL-8 in human epithelial cells in vitro (33). The overexpression of Fra-1 was reported to block the expression of LPS-induced inflammatory cytokines in a macrophage cell line, RAW264.7 (34). The overexpression of Fra-1 in mice also results in diminished levels of proinflammatory cytokine production in colon segments after the administration of dextran sulfate (9). However, in agreement with our results, the overexpression of Fra-1 reportedly led to enhanced susceptibility to LPS-induced mortality in mice (35). Although no data related to LPS-induced lung inflammation and mortality were indicated, the authors found no significant differences in the LPS-induced expression of inflammatory cytokines between wild-type and Fra-1–null mice at 12 hours after intraperitoneal treatment with LPS. Thus, the differences observed between these studies and ours can likely be attributed to differences in experimental and treatment conditions (e.g., the overexpression of Fra-1 versus the deletion of Fra-1, different time points of analysis, treatment with LPS versus treatment with dextran sulfate, a pulmonary environment versus a colonic environment, or the nature of primary cells versus immortalized cells).
NF-κB is a critical regulator of LPS-induced immune responses, and induces the up-regulation of most of the LPS-induced proinflammatory genes (36). The reduced expression of LPS-induced proinflammatory cytokines in Fra-1Δ/Δ lung tissue and macrophages (Figures 4 and 5) indicates that lower levels of NF-κB activation occur in Fra-1 mutant cells. Indeed, the NF-κB binding analyzed by a DNA binding ELISA assay revealed diminished levels of NF-κB binding in both the macrophages and lungs of Fra-1Δ/Δ mice treated with LPS, compared with their wild-type counterparts (Figure 6B). Thus, Fra-1 appears to augment LPS-induced inflammatory responses by increasing the activation of NF-κB. Although previous studies reported the involvement of a crosstalk between NF-κB and AP-1 family members (37), the exact mechanisms by which the loss of Fra-1 leads to reduced NF-κB activity in response to LPS remain unclear and in need of investigation.
In conclusion, our results show that a lack of Fra-1 decreases susceptibility to lethal challenge with LPS and also attenuates nonlethal LPS-induced inflammatory responses in the lung, illuminating a prominent role for Fra-1 in promoting LPS-induced lung injury and mortality. In addition, our results suggest that Fra-1 mediates its proinflammatory role through modulating the activation of c-Jun and NF-κB in response to exposure to LPS. Therefore, the development of strategies to target Fra-1 may be useful in inhibiting lung injury and inflammation in patients with ALI and ARDS syndromes, and in minimizing the effects of exposure to endotoxins in clinical settings.
Acknowledgments
The authors thank Dr. Erwin F. Wagner for the generous gift of the Fra-1 floxed mice used in our studies. The authors thank both the Pathology Core of the Acute Lung Injury-Specialized Centers of Clinically Oriented Research (ALI SCCOR) and the Microarray Core, supported by the Hopkins-National Institute of Environmental Health Sciences (NIEHS) Center, for the use of their facilities and services in the present study. The authors thank Alexis Bierman for assistance in mice handling and the instillation of LPS, Ashley Irving and Karinne Chevalier for technical assistance, and Dr. Deborah McClellan for editorial assistance. Note that the manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Footnotes
Narsa M. Reddy, Raja Subbiah, and Sekhar P. Reddy are currently at the Department of Pediatrics, University of Illinois at Chicago, M/C 856, 830 S. Wood St., Chicago, IL 60612.
This work was supported by National Institutes of Health grants ES11863 and HL66109 (S.P.R.) and by a Flight Attendant Medical Research Institute award (S.P.R. and M.V.).
M.V. and S.P.R. were involved in the conception, delineation of hypotheses, and design of the study, as well as the analysis and interpretation of data. M.V. performed the experiments. N.M.R. was involved in the acquisition of data. R.S. performed the ELISA assays.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0169OC on August 4, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693 [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001;1:135–145 [DOI] [PubMed] [Google Scholar]
- 3.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature 2000;406:782–787 [DOI] [PubMed] [Google Scholar]
- 4.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol 2007;178:6504–6513 [DOI] [PubMed] [Google Scholar]
- 5.Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol 2006;176:4995–5005 [DOI] [PubMed] [Google Scholar]
- 6.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 2003;3:859–868 [DOI] [PubMed] [Google Scholar]
- 7.Zenz R, Eferl R, Scheinecker C, Redlich K, Smolen J, Schonthaler HB, Kenner L, Tschachler E, Wagner EF. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther 2008;10:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray N, Kuwahara M, Takada Y, Maruyama K, Kawaguchi T, Tsubone H, Ishikawa H, Matsuo K. C-Fos suppresses systemic inflammatory response to endotoxin. Int Immunol 2006;18:671–677 [DOI] [PubMed] [Google Scholar]
- 9.Takada Y, Ray N, Ikeda E, Kawaguchi T, Kuwahara M, Wagner EF, Matsuo K. Fos proteins suppress dextran sulfate sodium–induced colitis through inhibition of NF-kappaB. J Immunol 2010;184:1014–1021 [DOI] [PubMed] [Google Scholar]
- 10.Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D, Scheuch H, Angel P, Tschachler E, Wagner EF. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 2005;437:369–375 [DOI] [PubMed] [Google Scholar]
- 11.Reddy SP, Mossman BT. Role and regulation of activator protein–1 in toxicant-induced responses of the lung. Am J Physiol Lung Cell Mol Physiol 2002;283:L1161–L1178 [DOI] [PubMed] [Google Scholar]
- 12.Cohen DR, Curran T. Fra-1: a serum-inducible, cellular immediate–early gene that encodes a Fos-related antigen. Mol Cell Biol 1988;8:2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 1997;9:240–246 [DOI] [PubMed] [Google Scholar]
- 14.Vaz M, Bierman AM, Reddy NM, Hassoun PM, Wagner EF, Reddy SP. Genetic disruption of Fra-1/AP-1 transcription factor decreases susceptibility to endotoxin-induced lung inflammation and death in mice. Am J Respir Crit Care Med 2010;181:A1801 (abstract) [Google Scholar]
- 15.Schreiber M, Wang ZQ, Jochum W, Fetka I, Elliott C, Wagner EF. Placental vascularisation requires the AP-1 component Fra1. Development 2000;127:4937–4948 [DOI] [PubMed] [Google Scholar]
- 16.Eferl R, Hoebertz A, Schilling AF, Rath M, Karreth F, Kenner L, Amling M, Wagner EF. The Fos-related antigen Fra-1 is an activator of bone matrix formation. EMBO J 2004;23:2789–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tallquist MD, Soriano P. Epiblast-restricted Cre expression in more mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis 2000;26:113–115 [DOI] [PubMed] [Google Scholar]
- 18.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene 2001;20:2390–2400 [DOI] [PubMed] [Google Scholar]
- 19.Lentsch AB, Ward PA. Regulation of experimental lung inflammation. Respir Physiol 2001;128:17–22 [DOI] [PubMed] [Google Scholar]
- 20.Strieter RM, Kunkel SL. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med 1994;42:640–651 [PubMed] [Google Scholar]
- 21.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev 2005;208:126–140 [DOI] [PubMed] [Google Scholar]
- 22.Roy S, Charboneau R, Cain K, DeTurris S, Melnyk D, Barke RA. Deficiency of the transcription factor c-Fos increases lipopolysaccharide-induced macrophage interleukin 12 production. Surgery 1999;126:239–247 [PubMed] [Google Scholar]
- 23.Matsumoto M, Einhaus D, Gold ES, Aderem A. Simvastatin augments lipopolysaccharide-induced proinflammatory responses in macrophages by differential regulation of the c-Fos and c-Jun transcription factors. J Immunol 2004;172:7377–7384 [DOI] [PubMed] [Google Scholar]
- 24.Schonthaler HB, Guinea-Viniegra J, Wagner EF. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis 2011;70:i109–i112 [DOI] [PubMed] [Google Scholar]
- 25.Rincon M, Derijard B, Chow CW, Davis RJ, Flavell RA. Reprogramming the signalling requirement for AP-1 (activator protein–1) activation during differentiation of precursor CD4+ T-cells into effector Th1 and Th2 cells. Genes Funct 1997;1:51–68 [DOI] [PubMed] [Google Scholar]
- 26.Hartenstein B, Teurich S, Hess J, Schenkel J, Schorpp-Kistner M, Angel P. Th2 cell–specific cytokine expression and allergen-induced airway inflammation depend on JunB. EMBO J 2002;21:6321–6329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meixner A, Zenz R, Schonthaler HB, Kenner L, Scheuch H, Penninger JM, Wagner EF. Epidermal JunB represses G-CSF transcription and affects haematopoiesis and bone formation. Nat Cell Biol 2008;10:1003–1011 [DOI] [PubMed] [Google Scholar]
- 28.Pflegerl P, Vesely P, Hantusch B, Schlederer M, Zenz R, Janig E, Steiner G, Meixner A, Petzelbauer P, Wolf P, et al. Epidermal loss of JunB leads to a SLE phenotype due to hyper IL-6 signaling. Proc Natl Acad Sci USA 2009;106:20423–20428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q, Kleeberger SR, Reddy SP. DEP-induced Fra-1 expression correlates with a distinct activation of AP-1–dependent gene transcription in the lung. Am J Physiol Lung Cell Mol Physiol 2004;286:L427–L436 [DOI] [PubMed] [Google Scholar]
- 30.Yoshioka K, Deng T, Cavigelli M, Karin M. Antitumor promotion by phenolic antioxidants: inhibition of AP-1 activity through induction of Fra expression. Proc Natl Acad Sci USA 1995;92:4972–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki T, Okuno H, Yoshida T, Endo T, Nishina H, Iba H. Difference in transcriptional regulatory function between c-Fos and Fra-2. Nucleic Acids Res 1991;19:5537–5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Ni H, Lan L, Wei X, Xiang R, Wang Y. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2D macrophages. Cell Res 2010;20:701–712 [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann E, Thiefes A, Buhrow D, Dittrich-Breiholz O, Schneider H, Resch K, Kracht M. MEK1-dependent delayed expression of Fos-related antigen–1 counteracts c-Fos and p65 NF-kappaB–mediated interleukin-8 transcription in response to cytokines or growth factors. J Biol Chem 2005;280:9706–9718 [DOI] [PubMed] [Google Scholar]
- 34.Morishita H, Saito F, Kayama H, Atarashi K, Kuwata H, Yamamoto M, Takeda K. Fra-1 negatively regulates lipopolysaccharide-mediated inflammatory responses. Int Immunol 2009;21:457–465 [DOI] [PubMed] [Google Scholar]
- 35.Takada Y, Gresh L, Bozec A, Ikeda E, Kamiya K, Watanabe M, Kobayashi K, Asano K, Toyama Y, Wagner EF, et al. Interstitial lung disease induced by gefitinib and Toll-like receptor ligands is mediated by Fra-1. Oncogene 2011;30:3821–3832 [DOI] [PubMed] [Google Scholar]
- 36.Blackwell TS, Blackwell TR, Christman JW. Impaired activation of nuclear factor–kappaB in endotoxin-tolerant rats is associated with down-regulation of chemokine gene expression and inhibition of neutrophilic lung inflammation. J Immunol 1997;158:5934–5940 [PubMed] [Google Scholar]
- 37.Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. NF-kappaB and AP-1 connection: mechanism of Nf-kappaB–dependent regulation of AP-1 activity. Mol Cell Biol 2004;24:7806–7819 [DOI] [PMC free article] [PubMed] [Google Scholar]