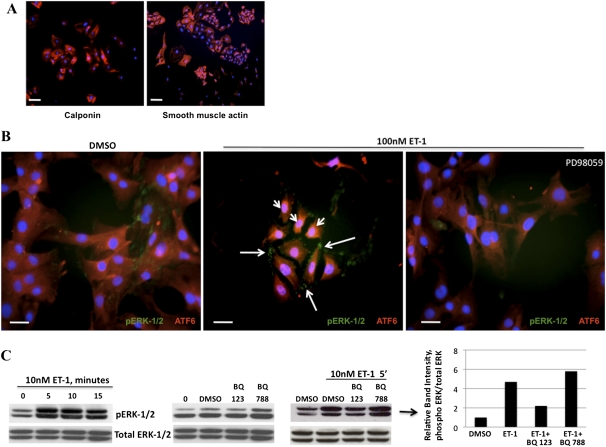
Figure 1.
Endothelin-1 (ET-1) induces the phosphorylation of extracellular signal–regulated kinase 1 and 2 (ERK-1/2) by pulmonary artery smooth muscle cells (PASMCs) and activation of the activating transcription factor 6 (ATF6) arm of the unfolded protein response (UPR). (A) Fluorescence microscopy of isolated PASMCs (calponin or smooth muscle actin = Cy3, 4',6-diamidino-2-phenylindole [DAPI] nuclei). (B) Within 5 minutes, ET-1 induced the rapid nuclear localization of the UPR protein ATF6 (arrowheads, Cy3), and also caused the phosphorylation (p) of ERK-1/2 (arrows, Alexa 488), both of which could be blocked by the mitogen activated protein kinase inhibitor PD98059. (C) Immunoblot time-course analysis of the phosphorylation of ERK-1/2 induced by ET-1, which peaked at 5–10 minutes. The phosphorylation of ERK-1/2 was blocked by the preincubation of PASMCs with the endothelin A receptor (ETA) receptor antagonist BQ123, but not the endothelin B receptor (ETB) receptor antagonist BQ788. Bar = 10 μm.