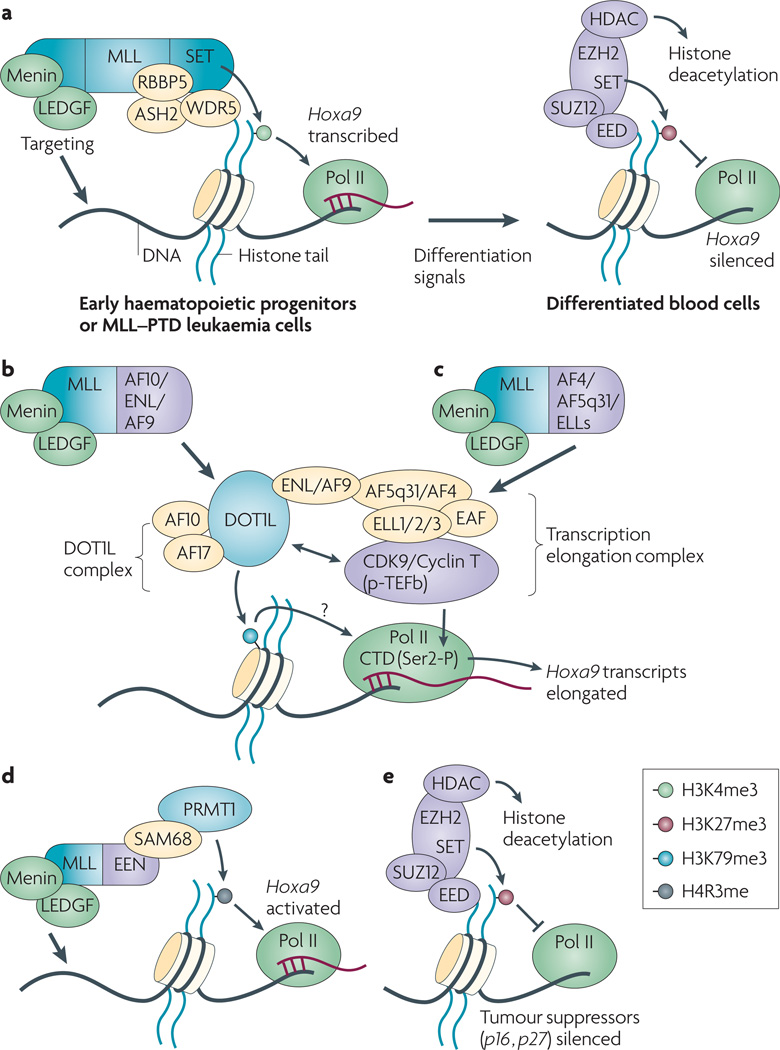
Figure 1. ‘Miswriting’ of histone methylation is associated with the initiation or progression of human cancer.
(a) MLL-containing complexes induce H3K4me3 at Hox genes in early hematopoietic progenitor cells. Following terminal differentiation, a transition of chromatin state occurs at Hox, which is characterized by loss of H3K4me3 and gain of EZH2-mediated H3K27me321, 66. EZH2 polycomb factors and associated HDACs induce the stable silencing of Hox. MLL-PTD, a MLL rearrangement form that harbors a duplication of MLL exon 4–12, causes an elevated level of H3K4 methylation.
(b–d) In leukemia, MLL fusion proteins lose a large carboxyl portion that includes the H3K4me3-‘writing’ SET domain, retain the chromatin targeting factors (Menin and LEDGF), and also acquire aberrant trans-activation mechanisms through its fusion partner. A subset of MLL fusions, MLL-AF10, MLL-ENL and MLL-AF9, directly interact with DOT1L and induce the methylation of H3K79 at Hoxa9 (panel b). Some other MLL fusions, MLL-AF4, MLL-AF5q31 and MLL- ELL1, interact with and recruit p-TEFb transcription elongation complexes to Hoxa9 (panel c). DOT1L-complexes (DOT1L-AF10-AF17-ENL/AF9) associate with p-TEFb complexes via the shared components. Another MLL fusion partner EEN recruits PRMT1 and induce methylation of H4R3 at Hox (panel d).
(e) Over-expression of EZH2 in tumor cells silences the tumor suppressor gene such as INK4B-ARF-INK4A. Please note that EZH2 can regulate oncogenes (panel a) or tumor suppressors (panel e) in different cellular contexts.