Figure 4.
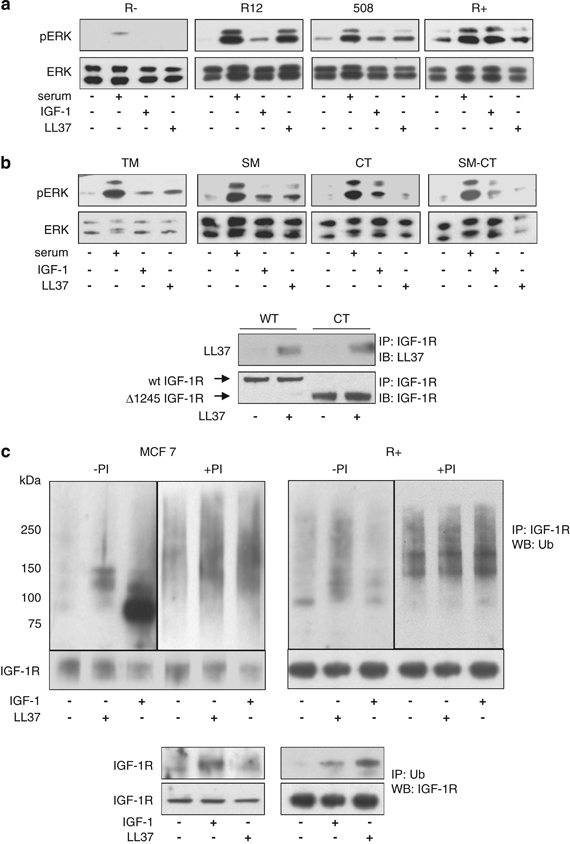
Mechanism of LL-37-induced ERK activation. Expression of IGF-1R is required for the activation of the MAPK/ERK pathway by LL-37. mouse embryonic fibroblast cells lacking IGF-1R (R−) or expressing increased numbers of human IGF-1R (R12, 508 and R+ cells) were serum-starved for 24 h and treated with serum, LL-37 or IGF-1 for 10 min and then assayed for ERK1/2 phosphorylation (a). LL-37-induced activation of ERK can occur independently of the tyrosine kinase signaling pathways TM (R− cells stably transfected with IGF-1R kinase defective triple mutant, where the activation domain tyrosine residues 1131, 1135 and 1136 are mutated to alanine), SM (R− stably transfected with IGF-1R with a substrate binding Y950 mutation to alanine), CT (R− stably transfected with IGF-1R and lacking the C-terminal), and SM-CT (R− stably transfected with IGF-1R with a Y950A mutation and lacking the C-terminal), cells were serum-depleted for 24 h and then stimulated as described in panel a (b). The LL-37 binding to the Δ1245 IGF-1R was verified by using the CT cells. Wild-type or CT cells were cultured overnight in the absence of serum and then treated or not for 10 min with 9 μg/ml LL-37. IGF-1R was immunoprecipitated from total protein lysates with an anti-IGF-1R antibody and the levels of LL-37 binding were assayed by western blot with an anti-LL-37 antibody. As a loading control, the total levels of IGF-1R were determined using an antibody against IGF-1R (b, lower panel). Effects of LL-37 stimulation on IGF-1R ubiquitination. MCF-7 and R+ cells were serum depleted for 24 h and then either untreated or treated with the proteasome inhibitor MG132 (PI) for 1 h before 10-min stimulation with IGF-1 or LL-37. IGF-1R was immunoprecipitated from the cell lysates and equal amounts of immunoprecipitates were fractionated and probed with an anti-ubiquitin antibody. Detection of the IGF-1R was used as a loading control (upper panel). IGF-1R ubiquitination in both MCF-7 and R+ cell lines was also verified by immunoprecipitating the cell lysates with an antibody to ubiquitin (P4D1) and immunoblotting with an antibody recognizing IGF-1R (lower panel) (c).
