Abstract
The human gamma (γ)-herpesviruses Epstein-Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) establish lifelong latent infections, can reactivate in immunocompromised individuals, and are associated with the development of malignancies. Murine γ-herpesvirus-68 (γHV68), a rodent pathogen related to EBV and KSHV, provides an important model to dissect mechanisms of immune control and investigate vaccine strategies. Infection of mice with γHV68 elicits robust antiviral immunity, and long-term protection from γHV68 reactivation requires both cellular and humoral immune responses. Vaccination of mice with AC-RTA, a highly lytic latency-null recombinant γHV68, results in complete protection from wild-type γHV68 infection that lasts for at least 10 months. In this report, we examine the immune correlates of AC-RTA-mediated protection and show that sterilizing immunity requires both T cells and antibody. Importantly, antibody was also critical for mitigating viral infection in the brain and in the absence of antibody-mediated control, amplification of the AC-RTA virus in the brain resulted in fatality. Our results highlight important considerations in the development of vaccination strategies based on live-attenuated viruses.
Introduction
The human gamma (γ)-herpesviruses, Epstein-Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) are widely-disseminated pathogens that establish life-long latent infections requiring constant immune surveillance. Latent infection is largely benign in the majority of infected individuals, and it has been demonstrated that γ-herpesvirus latency can be beneficial to the host by enhancing resistance to bacterial infections (1). However, the viruses have oncogenic potential and infection is associated with the development of malignancies in immunosuppressed individuals and specific high risk groups. For example, post-transplant lymphoproliferative diseases are a well-known consequence of EBV in immunosuppressed transplant recipients, and both KSHV and EBV cause a high rate of malignancy in AIDS patients. In addition, regions of Southeast Asia have a 20-fold higher than normal incidence of EBV-associated nasopharyngeal carcinoma, and EBV is associated with a high frequency of Burkitt’s lymphoma in areas of holoendemic malaria in equatorial Africa (2, 3). Thus, the development of vaccines capable of preventing the establishment of γ-herpesvirus latency will lower the risk of associated malignancies. Because of the species specificity of the γ-herpesviruses, EBV and KSHV can’t be directly used in a mouse model. However, the mouse γ-herpesvirus γHV68 is highly homologous to EBV and KSHV, and mechanisms of immune control are similar (4-6). Importantly, γHV68 provides a natural in vivo infection model in which host immune evasion mechanisms are preserved.
Many important principles have been established by vaccination studies using the mouse γHV68 model. Early vaccination strategies that focused on subunit and inactivated viral vaccines met with limited success. For example, similar to results in human trials (7), strategies targeting γHV68 gp150 (the homolog of EBV gp350) were shown to lessen infectious mononucleosis-like symptoms, but failed to impact the establishment of long-term latency (8). In addition, dominant lytic and latent epitopes that have been identified in the mouse model were used in targeted vaccination strategies. Although vaccination with well-characterized lytic T cell epitopes reduced the extent of lytic infection upon challenge, this strategy failed to prevent the establishment of latency and did not substantially lower the long-term latent viral load (9, 10). This is consistent with accumulating data showing that latency can be established independent of the lytic infection (11-14), which explains why targeting the lytic phase of infection does not significantly impact the establishment of latency. Subunit vaccines that directly target the establishment of latency were also unsuccessful, as vaccination with a defined latency epitope yielded only transient protection (15, 16). Recent vaccination studies have shown only partial protection with live attenuated mutant viruses incapable of replication or reactivation (14, 17-19). Because of the oncogenic nature of the γ-herpesviruses, engineered viruses that are incapable of establishing latency hold the greatest promise for successful and safe vaccine candidates (20-22).
We have recently generated a live-attenuated virus, termed AC-RTA, that fails to establish latency and elicits strong and long-lasting protection against infection with wild-type virus (23). A dual strategy was used to generate AC-RTA. First, the γHV68 genome was modified to allow constitutive over-expression of the viral replication and transcription activator, RTA, which drives the virus preferentially into the lytic cycle. Second, viral sequences necessary for the establishment of latency and oncogenicity were deleted, including ORF73 (a latency-associated nuclear antigen, LANA, homolog), ORF72 (v-cyclin) and M11 (a viral bcl-2 homolog). AC-RTA exhibits enhanced lytic replication demonstrated by larger plaque size in vitro, higher peak viral titers in vivo and faster clearance from immunocompetent mice (23). We previously showed that infection of BALB/c mice with AC-RTA generated a strong lytic infection in the lung that was efficiently cleared by the immune system. Although the virus failed to establish latency, strong protective immunity was generated that prevented subsequent infection with wild type virus (23); both virus-specific T cells and antibodies were induced (23, 24). In the current studies we examined the vaccination efficacy of AC-RTA in C57BL/6 mice and determined the immune correlates of protection. Consistent with the finding that both humoral and cellular immunity are required to control latent γHV68 infection, we found that both antibody and T cells contribute to protective immunity induced by AC-RTA. Unexpectedly however, we found that antibody-deficient mice failed to control AC-RTA infection, and died with high viral titers in the brain.
Materials and Methods
Mice and virus infections
Female 6- to 12-week-old C57BL/6, AID−/−, μs−/−, AID−/−μs−/−, B6.129S2-Igh-6tm1Cgn (μMT), and B6.129S-H2dlAb1-Ea (MHC class II−/−) mice were obtained from the Trudeau Institute animal facility and maintained in specific-pathogen-free conditions. Mice were infected intranasally (i.n.) with 400 PFU or intraperitoneally (i.p.) with 104 PFU γHV68 (strain WUMS) or AC-RTA (23). All animal studies were approved by the Institutional Animal Care and Use Committee of the Trudeau Institute.
Plaque assays
Plaque assays were performed as described previously (25). In short, spleens, lungs, and brains were homogenized, serially diluted, and plated on NIH3T3 cell monolayers for 1.5 h. Homogenates were then removed and overlayed with 0.75% carboxymethylcellulose in media. Plates were incubated for 6 d at 37°C, 10% CO2. Cells were then fixed with methanol, stained with Giemsa violet stain, and plaques were counted.
Flow cytometry
Splenocytes were treated with Fc Block then stained with antibody against CD8α (BD Biosciences) and allophycocyanin-conjugated MHC-I tetramers specific for γHV68 epitopes H-2Db/ORF6487-495 (AGPHNDMEI) or H-2Kb/ORF61524-531 (TSINFVKI) (obtained from the Trudeau Institute Molecular Biology Core Facility). Samples were collected on a BD FACSCanto II cytometer and analyzed using FlowJo software (TreeStar).
Enzyme-linked immunosorbent assay (ELISA)
ELISAs for virus-specific IgG were performed as described previously (26). In short, plates were coated with γHV68 virions (0.5 μg/well), incubated with four-fold serially diluted sera from experimental or control mice, then incubated with appropriate alkaline phosphatase-conjugated goat anti-mouse antibody (Southern Biotech). Plates were then washed and incubated with p-nitrophenyl phosphate substrate. Optical density was then measured at 405 nm; optical density values higher than twice the naïve serum samples were considered a positive reaction.
Serum transfers
Serum was harvested from whole blood collected from mice at 1 or 4 m after γHV68 infection or AC-RTA vaccination. To test the protective efficacy of immune serum, naïve mice were treated i.p. with 250 μl serum one day prior to infection with γHV68. Lungs were harvested at 3 and 7 d p.i. and analyzed by plaque assays.
In vitro neutralizing antibody assay
The neutralizing activity of serum antibody was assessed in vitro as described previously, with slight modifications (27). Briefly, heat-inactivated serum samples were serially-diluted two-fold in media and mixed 1:1 with 103 PFU/ml γHV68 for 1.5 h at 37°C, then overlayed on NIH3T3 cell monolayers for plaque assays.
In vivo T cell depletion with antibody
T cell subsets were depleted from naïve or AC-RTA-vaccinated mice by treatment with monoclonal antibodies to CD4 (GK1.5), CD8 (2.43), or anti-Thy1.2 (30H12) (BioXcell). For each group, 250 μg of antibody was administered i.p. every 2-3 d beginning 2 d prior to γHV68 challenge. Lungs and spleens were harvested at 7 d p.i. and analyzed by plaque assays.
Quantitative real-time PCR
DNA was isolated from homogenized brain tissue using the DNeasy Mini kit (Qiagen) and quantified with a Nanodrop ND-1000 spectrophotometer (ThermoFisher Scientific). The copy number of the γHV68 ORF50 gene in 200 ng DNA was determined by quantitative real-time PCR essentially as described (16), using a standard curve quantitation method on an Applied Biosystems 7500 Real-Time PCR System, using Taqman Gene Expression master mix (Applied Biosystems). Primers, probes, and reaction cycles used are as described in Usherwood, et al (16).
Statistical Analysis
Data were analyzed using Student’s t test, one-way ANOVA, or Log-Rank (Mantel-Cox) test for survival curves. All analyses were performed using Prism 5 software (GraphPad).
Results
AC-RTA confers long-lasting protection following vaccination
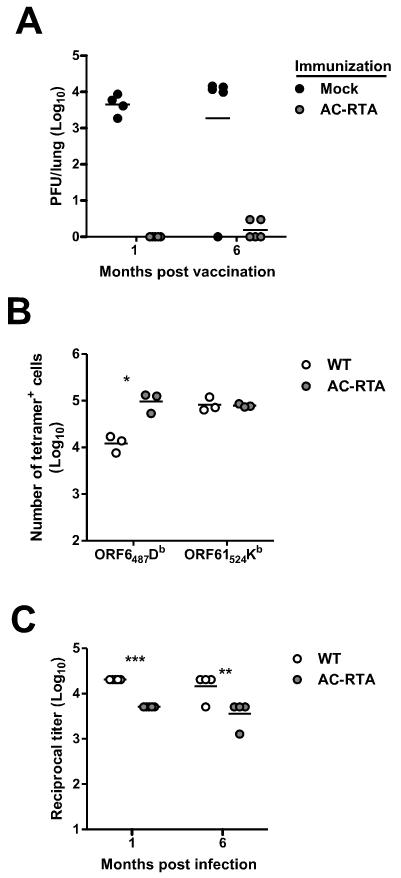
Our previous study examined protection from wild-type γHV68 challenge in BALB/c mice up to 3 months after vaccination with AC-RTA (23). To determine if AC-RTA vaccination induced long-lasting protection in C57BL/6 mice, we intranasally vaccinated mice with AC-RTA, challenged them intranasally with wild type (WT) γHV68 either 1 or 6 months post vaccination, and examined viral titers in the lungs 7 days after challenge. As shown in Figure 1A, AC-RTA vaccination of C57BL/6 mice was highly protective against WT virus challenge, with protection lasting for at least 6 months.
Figure 1. Long-lasting protection following AC-RTA vaccination.
A, The number of PFU per lung of AC-RTA- or mock-immunized mice 7 d after challenge with WT γHV68. B, The number of tetramer-positive CD8 T cells in the spleen, 7 m after WT γHV68 infection or AC-RTA vaccination. *P≤0.05, Student’s t test. C, Reciprocal titers of virus-specific IgG antibody 6 m after WT γHV68 infection or AC-RTA vaccination. **P≤0.01, ***P≤0.001, Student’s t test.
We next examined the cellular and humoral immunity elicited by AC-RTA vaccination compared to WT virus infection (Figure 1B). We have previously examined virus-specific CD8 T cell responses in the spleen after infection with AC-RTA and WT virus. As we previously reported, numbers of tetramer-positive cells for two different epitopes, ORF6487/Db (ORF6) and ORF61524/Kb (ORF61) were comparable 12 days p.i. with either virus, and one month after infection, ORF61-specific cells, but not ORF6-specific cells, were reduced in the absence of latency amplification (24). Here we examined tetramer levels 7 months after WT infection or AC-RTA vaccination (Figure 1B). Although ORF61-specific cells are normally maintained long-term at a higher level than ORF6-specific cells after WT virus infection, in the absence of latency both pools of epitope-specific T cells were maintained comparably. This shift in immunodominance is similar to a previous report using a different latency-deficient recombinant virus (28), and implicates latency or reactivation from latency in the preferential maintenance of ORF61-specific T cells.
We have previously shown that the levels of virus-specific IgG elicited by WT virus infection of C57BL/6 mice are maintained over the long-term (22 months), but neutralizing titers progressively decline (29). Therefore, we examined virus-specific antibody responses and found that both WT virus and AC-RTA vaccination elicited robust class-switched antibody responses that were sustained as long as 6 months p.i. as measured by ELISA, although AC-RTA vaccination elicited lower antibody titers than WT γHV68 infection (Figure 1C). Together, the data show that vaccination with AC-RTA provides long-lasting protection from WT viral challenge in C57BL/6 mice, and induces sustained cellular and humoral immunity.
Both virus-specific T cells and antibody contribute to protection
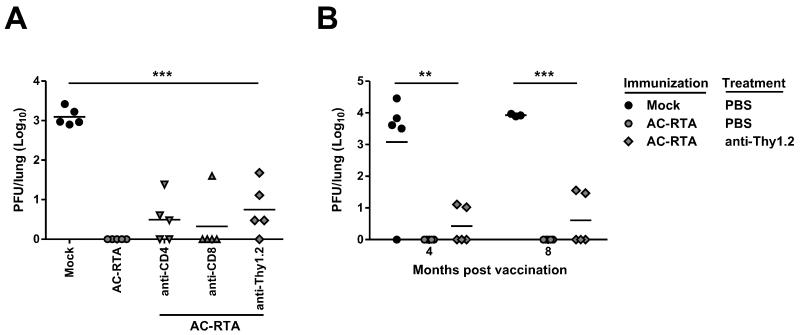
To determine the relative roles of antiviral CD4 and CD8 T cells in AC-RTA vaccine-mediated protection, mice were depleted of CD4 T cells, CD8 T cells, or both by monoclonal antibody administration 30 days after vaccination with AC-RTA, beginning 2 days prior to WT virus challenge. Protection was assessed by monitoring the presence of WT γHV68 in the lung 7 days after challenge (Figure 2A). Depletion of T cells resulted in reduced vaccine-mediated protection, with indications of a greater role for CD4 T cells than CD8 T cells (2 out of 5 fully protected after anti-CD4 treatment versus 4 out of 5 fully protected after anti-CD8 treatment), although there was variation between individual mice. However, even total T cell depletion did not completely abrogate protection, suggesting a role for antibody. Challenge of mice depleted of T cells 4 or 8 months after AC-RTA vaccination again resulted in partial loss of protection (Figure 2B), consistent with a role for both T cells and antibody in long-term protection.
Figure 2. Contribution of T cells to AC-RTA-mediated protection.
A, The number of PFU per lung 7 d after WT γHV68 challenge of mock-vaccinated mice, mice vaccinated 30 d prior with AC-RTA, or AC-RTA-vaccinated mice treated with anti-CD4, anti-CD8, or anti-Thy1.2 mAb prior to challenge. ***P≤0.001, one way ANOVA. B, The number of PFU per lung 7 d after WT γHV68 challenge of mock-vaccinated mice, mice vaccinated 4 or 8 m prior with AC-RTA, or AC-RTA-vaccinated mice treated anti-Thy1.2 mAb prior to challenge. **P≤0.01, ***P≤0.001, one way ANOVA.
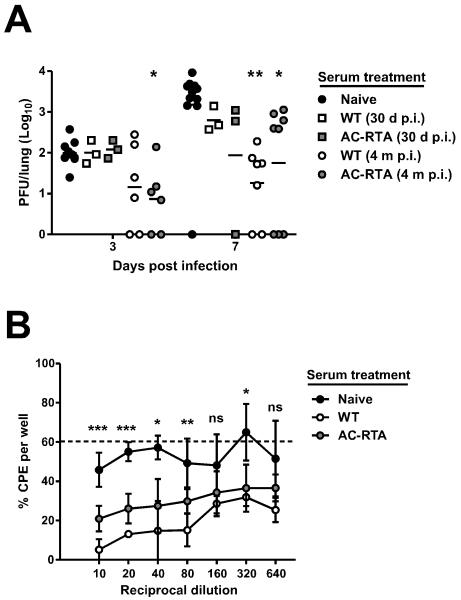
Since antibody was playing a role in protection, we addressed the contribution of antiviral antibody in two ways. First, we passively transferred WT- or AC-RTA-immune serum into naïve mice, then challenged with WT virus (Figure 3A). Sera from both WT- or AC-RTA-infected mice were protective compared to naïve serum. A previous report has shown that the class-switched and neutralizing antibody response to γHV68 rises rapidly the first three weeks after infection and continues to increase over several months (27). Consistent with that report, we found that serum from WT-immune mice 4 months p.i. provided greater protection than serum from WT-immune mice 30 days after infection, especially when protection was measured 7 days after challenge. AC-RTA-immune serum did not exhibit the same increase in protective efficacy over time after vaccination – AC-RTA-immune serum 4 months p.i. elicited significantly less protection 7 days after challenge than WT-immune serum from mice 4 months p.i. (P=0.0415, Student’s t test), consistent with the lower overall antiviral IgG after AC-RTA vaccination compared to WT γHV68 infection (Figure 1C). Second, we compared the ability of serum obtained from WT- and AC-RTA-infected mice 4 months after infection to neutralize virus in an in vitro assay (Figure 3B). At each dilution, serum from WT-immune mice 4 months p.i. had more neutralizing activity than serum from AC-RTA-vaccinated mice, although both sera neutralized γHV68 more effectively than naïve serum. The enhanced protective efficacy of serum from mice infected with WT virus raises the possibility that the presence of latent virus drives maturation of the neutralizing response. The possibility that the antibody-specific response generated after vaccination by vectors that do not induce latency is deficient may have important implications for vaccination efficacy in cases of declining T cell function.
Figure 3. Contribution of antibody to AC-RTA-mediated protection.
A, The number of PFU per lung 3 or 7 d after WT γHV68 challenge in mice treated with sera from naïve mice, mice 30 d or 4 m after WT infection, or mice 30 d or 4 m after AC-RTA vaccination. *P≤0.05, **P≤0.01, one way ANOVA. B, The percent of wells positive for cytopathic effect (CPE) after in vitro infection with WT γHV68 pre-treated with naïve serum, or sera from mice 4 m after WT infection or AC-RTA vaccination (n=2-5/group). Dotted line is at 60.3%, the average percent of CPE when no serum was added. ns, not significant, *P≤0.05, **P≤0.01, ***P≤0.001, one way ANOVA.
Taken together, our data show that T cells and antibody are both influenced by the presence of latent virus. Importantly, each contributes to the long-lasting protection afforded by AC-RTA vaccination.
Antibody-deficient mice succumb to AC-RTA vaccination
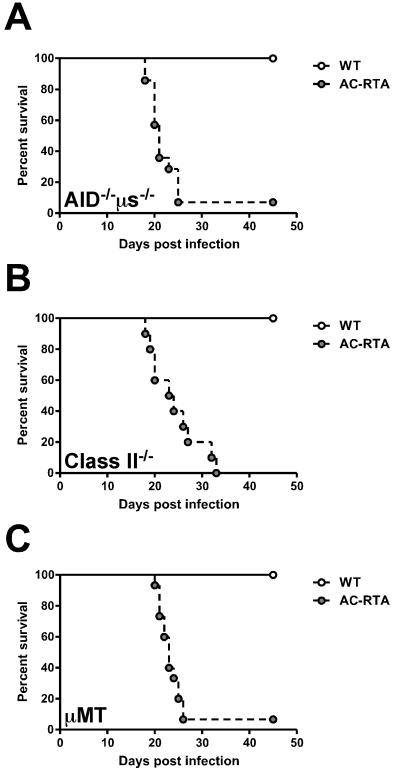
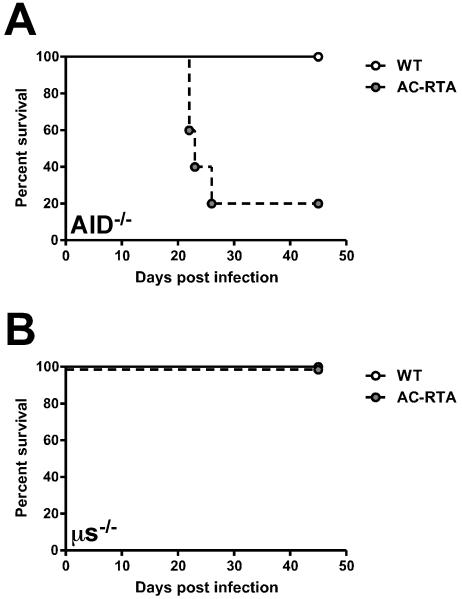
To further investigate the role for antibody in AC-RTA vaccine-mediated protection in vivo, we infected antibody-deficient activation-induced cytidine deaminase (AID)/secretory μ-chain (μs) double-knockout mice (AID−/−μs−/−) with AC-RTA (30, 31). These mice are deficient in class-switching, somatic hypermutation, and IgM secretion, thereby lacking circulating antibodies while still retaining expression of immunoglobulin on the B cell surface. These mice maintain a complete B cell compartment, with the exception of plasma cells (30). Because B cells are the major reservoir of latent virus (32, 33), these mice are an excellent model for assessing the importance of humoral immunity in AC-RTA-mediated protection. To this end, C57BL/6 and AID−/−μs−/− mice were infected with WT and AC-RTA. Unexpectedly, at around 3 weeks, the AID−/−μs−/− mice succumbed to AC-RTA infection (Figure 4A). To determine if lethality was a characteristic unique to this particular strain of mouse, we infected two additional strains of antibody-deficient mice, MHC Class II−/− mice (Figure 4B) and B cell-deficient μMT mice (Figure 4C). All three strains succumbed to AC-RTA infection with similar kinetics, confirming a requirement for antibody in survival following AC-RTA infection.
Figure 4. Requirement for antibody during AC-RTA infection.
A, Survival of AID−/−μs−/− mice after WT γHV68 or AC-RTA i.n. infection (n=5-14/group, P=0.0013). B, Survival of MHC Class II−/− mice after WT γHV68 or AC-RTA i.n. infection (n=6-10/group, P=0.0002). C, Survival of μMT mice after WT γHV68 or AC-RTA infection (n=15/group, P≤0.0001).
Since AID−/−μs−/− mice cannot undergo class-switching or secrete IgM, we were unsure whether circulating IgM could prolong survival of antibody-deficient mice after AC-RTA infection. We infected AID−/− mice, which lack class-switched antibody but can still secrete IgM (34), and μs−/− mice, which cannot secrete IgM, but can still generate and secrete other antibody classes (35), with AC-RTA. AID−/− mice succumbed to AC-RTA infection with similar kinetics as above (Figure 5A) while μs−/− mice survived infection (Figure 5B). These data show that circulating IgM is not sufficient to allow survival of AC-RTA-infected mice lacking class-switched antibodies.
Figure 5. Class-switched antibody is required during AC-RTA infection.
A, Survival of AID−/− mice after WT γHV68 or AC-RTA i.n. infection (n=4-5/group, P=0.0249). B, Survival of μs−/− mice after WT γHV68 or AC-RTA i.n. infection (n=5-6/group).
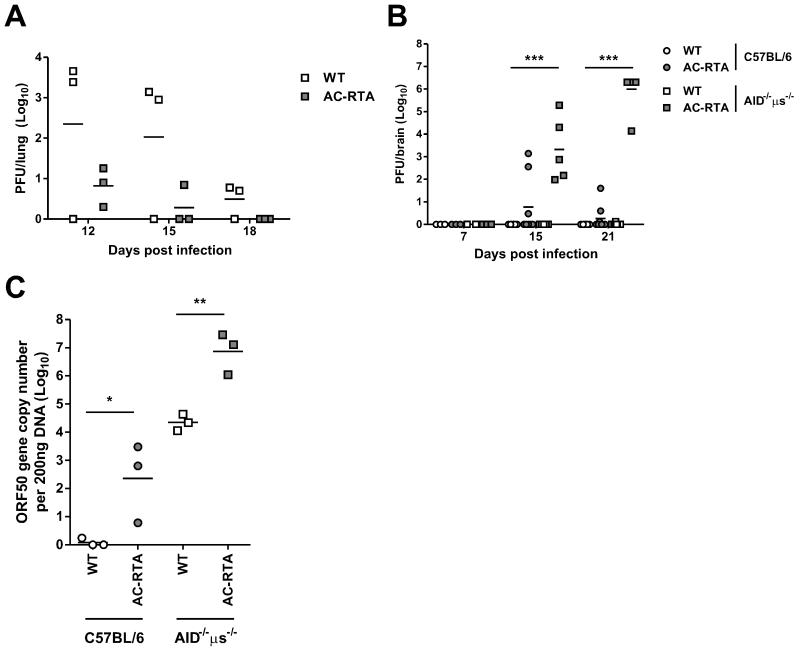
AC-RTA is biased toward lytic rather than latent infection because it has an ectopic copy of RTA driven by a strong hybrid promoter (23). Consistent with enhanced lytic replication, AC-RTA generates larger plaques and enhanced viral titers in multiple-step growth experiments in vitro (23). In addition, we have previously shown that AC-RTA infection of C57BL/6 mice results in higher and more rapid titers in the lung compared with WT infection (24). Therefore, we asked whether elevated AC-RTA titers could account for the death of AID−/−μs−/− mice. Whereas C57BL/6 mice clear both WT and AC-RTA virus by day 10 after intranasal infection (24), analysis of viral clearance in the lungs of AID−/−μs−/− mice showed impaired clearance of both viruses in the absence of antibody. Interestingly, AC-RTA infection was cleared with faster kinetics than WT virus in AID−/−μs−/− mice (Figure 6A), ruling out uncontrolled virus replication in the lungs as a cause of death in the mice.
Figure 6. Virus in the brain after AC-RTA infection of antibody-deficient mice.
A, The number of PFU per lung of AID−/−μs−/− mice at the indicated times after WT γHV68 or AC-RTA infection. B, The number of PFU per brain of C57BL/6 or AID−/−μs−/− mice at the indicated times after WT γHV68 or AC-RTA infection. ***P≤0.001, one way ANOVA. C, The number of viral ORF50 gene copies per 200 ng DNA from brains of C57BL/6 or AID−/−μs−/− mice 21 d after WT γHV68 or AC-RTA infection. *P≤0.05, **P≤0.01, Student’s t test.
Mice that succumbed to AC-RTA infection were moribund, exhibiting severe weight loss, ataxia, and hind-limb paralysis, consistent with the mice undergoing encephalitis. Therefore, we measured the lytic viral load in the brains of C57BL/6 and AID−/−μs−/− mice following infection with WT γHV68 or AC-RTA (Figure 6B). Remarkably, high titers of lytic virus were found in all the brains of AID−/−μs−/− mice on days 15 and 21 post AC-RTA infection, while only 1 out of 9 WT γHV68-infected AID−/−μs−/− mice had a detectable, but still very low, amount of virus. At no time did we detect lytic WT γHV68 in the brains of C57BL/6 mice. In antibody-deficient mice, WT γHV68 infection produced high levels of viral DNA in the brain, indicating that antibody plays a major role in preventing viral spread to the brain, but the virus in the brain is mostly maintained in a latent state (Figure 6C). Regardless of antibody, AC-RTA infection led to significant levels of viral DNA in the brain, consistent with the highly lytic nature of AC-RTA; once AC-RTA reaches the brain, it undergoes lytic replication that is mitigated by antibody. Taken together, in both WT γHV68 and AC-RTA infections, antibody is essential for controlling viral invasion of the brain and the importance of this antibody-mediated control is heightened by highly lytic AC-RTA infection, which causes antibody-deficient mice to die.
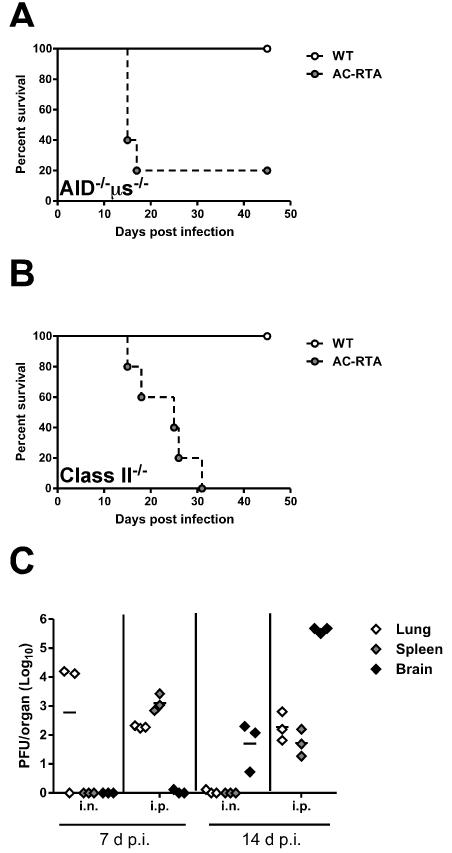
We next examined whether the infiltration and expansion of AC-RTA in the brain of AID−/−μs−/− mice was a consequence of the route of inoculation. It has been shown that following intranasally-administered AC-RTA that a high concentration of virus can be detected at the site of inoculation (23), raising the possibility that this facilitates localization to and infection of the brain. To address this, we intraperitoneally (i.p.) infected AID−/−μs−/− mice (Figure 7A) or MHC class II−/− mice (Figure 7B) with AC-RTA and measured survival. AID−/−μs−/− mice infected with AC-RTA i.p. succumbed to infection with faster kinetics than i.n. infected mice (compare Figure 7A with Figure 4A - median survival: i.p. 15 d, i.n. 21 d; P=0.0139), whereas MHC class II−/− mice succumbed with similar kinetics (compare Figure 7B with Figure 4B - median survival: i.p. 25 d, i.n. 23.5 d; P=0.6177). In either case, AC-RTA resulted in death in both strains following i.p. infection, conclusively demonstrating that AC-RTA is lethal to antibody-deficient mice regardless of the route of infection.
Figure 7. AC-RTA is lethal in antibody-deficient mice by i.p. infection route.
A, Survival of AID−/−μs−/− mice after WT γHV68 or AC-RTA i.p. infection (n=3-5/group, P=0.0453). B, Survival of MHC Class II−/− mice after WT γHV68 or AC-RTA i.p. infection (n=3-5/group, P=0.0136). C, The number of PFU per lung, spleen, or brain in AID−/−μs−/− mice after i.n. or i.p. infection with AC-RTA.
We next measured lytic viral titers in the lungs, spleens, and brains of AID−/−μs−/− mice following i.n. or i.p. infection with AC-RTA to determine if i.p. infection resulted in increased viral loads in the brain (Figure 7C). Interestingly, i.p. infection led to accelerated dissemination to the spleen compared with i.n. infection at 7 d p.i., but lytic virus could not be detected in the brain until 14 d p.i. The reasons for the delayed kinetics of virus in the brain compared with the spleen remain unclear.
Taken together, our data emphasize the importance of antibody responses in a recombinant viral vaccine strategy. In immunocompetent animals, AC-RTA vaccination leads to long-lasting protection from viral challenge that is dependent on both cellular and humoral immunity. In antibody-deficient animals, however, the highly lytic AC-RTA infection results in the infiltration and replication of the virus in the brain, correlating with encephalitis-like symptoms, morbidity, and death.
Discussion
This study has examined the immune mechanisms by which AC-RTA, a latency-deficient γHV68, induces long-lasting protective immunity against infection with WT virus. The data show that both T cells and antibody contribute to protection. Unexpectedly, we found that not only did antibody make an important contribution to immune protection after vaccination, but antibody was also essential for controlling the viral spread to the brain. This importance is highlighted by the lethality of highly lytic AC-RTA infection in antibody-deficient mice, which correlated with the presence of high viral titers in the brain. This finding was unexpected since i.n. infection of mice with WT γHV68 leads to a robust acute infection in the lungs followed by the establishment of viral latency in dendritic cells, macrophages, and B cells in the spleen and elsewhere (32), but γHV68 is not normally neuro-invasive after infection. γHV68 can infect the CNS if inoculated intracerebrally, however (36, 37). Additionally, i.n. inoculation with WT virus can lead to infection in the brains of immunocompromised mice, including neonatal BALB/c mice (38) and adult mice deficient in type I interferon receptor (36). These observations are also consistent with a study using an HSV vaccination model where it was shown that antibody and T cells elicited by a mutant replication-deficient vaccinating virus contributed to protection of the host at distinct stages of challenge infection, with antibody preventing encephalitic infection of the CNS (39). Therefore, our studies validate the importance of the mouse model for examining vaccine strategies and understanding correlates of immune protection, and raise a caution that live vaccines that are attenuated and protective in an immunocompetent host may have unanticipated virulence in immunocompromised individuals.
It has long been known that antibody participates in immunity to γHV68 (17, 26, 40). Two separate questions are raised by these studies – what is the role of antibody in protection elicited by vaccination with AC-RTA, a latency-deficient virus, and what is the role of antibody in control of the initial virus infection?
The observation that T cell depletion lowered the protective efficacy following vaccination is similar to previous observations using a different live attenuated virus that was capable of establishing latency but was incapable of reactivation. In agreement with our findings, it was shown that protection could be partially reconstituted by passive transfer of antibody (17). These earlier studies also suggested that antibody-mediated protection was not mediated by the simple blocking of the initial infection because vaccination was effective against high challenge doses (17). This conclusion is consistent with our data showing that passively transferred sera had only modest protection in terms of viral titers at day 3 (and thus did not prevent initial infection), but showed greater than one log reduced viral titers at day 7 (Figure 3A).
Our data show that passive transfer of serum from WT mice obtained four months post infection is more protective than antibody obtained from mice 30 d p.i. (Figure 3A). In contrast, serum from AC-RTA vaccinated mice is equally protective over time, consistent with the possibility that antibody elicited by AC-RTA infection failed to undergo some aspects of antibody maturation. It is possible that differences in protective efficacy reflect differences in neutralizing activity, as assessed in an in vitro assay (Figure 3B). However, the contribution of virus neutralizing antibody in protection is poorly understood. The observation that the protective efficacy of serum matures over time in WT γHV68-infected but not vaccinated mice raises the interesting possibility that latency or reactivation is necessary to drive this unknown aspect of antibody maturation, as AC-RTA vaccination fails to establish latency. The differences in protective antibody are not simply a consequence of class switching, as our analysis failed to reveal any statistically significant differences in isotype profile as late as 6 months after infection (data not shown). Differences in protection may reflect a change in specificity of virus-specific antibodies associated with different phases of infection, as complex serological patterns of antibodies specific for different viral proteins have been described during EBV latency, following reactivation, and associated with tumors (41).
It has been reported that human γ-herpesviruses can infect the central nervous system. Acute or reactivating infection with EBV in both immunocompromised as well as immunocompetent individuals has been linked to complications such as meningitis and encephalitis (42, 43). In addition, it has been shown that KSHV can be neuro-invasive (44). Neurological complications of KSHV infection are less well-defined, but detection of virus in dorsal root ganglia, cerebrospinal fluid and brain in some patients with central nervous system complications associated with Kaposi’s sarcoma or AIDS-associated dementia have been described, although the relative contributions of HIV and KSHV to the pathology is controversial (43, 44). It is unclear whether pathogenesis is a consequence of direct virus invasion of the CNS or whether damage is due to immunological effector mechanisms mediated either by infiltrating CD8 T cells, or deposition of antigen-antibody complexes (43).
Our data show that both the absence of antibody and the enhanced lytic activity of AC-RTA contribute to high viral titers in the brain following AC-RTA infection. Analysis of infectious virus and viral genome copy number in the brain has allowed us to distinguish the relative roles of enhanced lytic activity of AC-RTA and the absence of antibody in the inability of the antibody-deficient mice to control AC-RTA infection within the brain. Whereas AC-RTA infection of both antibody-deficient and WT mice resulted in lytic virus in the brain, the titers rose dramatically with time in the antibody-deficient mice and the mice succumbed to infection, but titers stabilized in the WT mice with no lethality. In contrast, no lytic virus was detected in the brains of either WT or antibody-deficient mice after infection with WT γHV68, but analysis of genome copy number was consistent with the presence of latent virus in the brain of WT γHV68-infected antibody-deficient mice. Taken together, these data show that both WT γHV68 and AC-RTA can infect the brain in the absence of antibody and that the antibody is essential to control viral infection of the brain. However, due to the highly lytic replication of AC-RTA, which contains an ectopic copy of RTA under the control of a strong and constitutive hybrid promoter to drive the viral lytic cycle, an unchecked AC-RTA infection in the brain of antibody-deficient mice leads to death. How might antibody prevent the robust replication of AC-RTA in the brains of WT mice? One intriguing possibility for antibody control of AC-RTA replication in the brain is through the activity of complement factor C3, as it has been shown that C3 can mediate γHV68 replication during direct CNS infections (45). Alternatively, antibody neutralizing activity could prevent the spread of lytic virus within the brain, possibly by Fc-receptor-mediated engulfment by phagocytes.
How does the virus get to the brain? In the absence of direct infection of the CNS, virus could be transported to the brain via cell-free virus in plasma or in a cell-associated manner. Our data show that both i.n. and i.p. infections lead to dissemination of virus to the brain by 14 d p.i., whereas the lytic titers in the brain are 3-logs higher following i.p. infection. Intriguingly, in vitro AC-RTA infection leads to the over-expression of the gene that encodes the viral regulator of complement activation (RCA) protein (23, 46), which has recently been shown to enhance virus replication within macrophages (47). Macrophages can acquire virus, especially after i.p. infection (48), suggesting infected macrophages may carry virus to the brain. Enhanced macrophage infection and trafficking of AC-RTA could also explain the higher titers of lytic virus in the brain after i.p. infection compared to i.n. infection. Antibody could play a role in preventing this trafficking, but this antibody-dependent prevention might be overcome by the over-expression of RCA after AC-RTA infection.
Together, these studies have provided important insight into mechanisms of γ-herpesvirus immune control and immune correlates of protection elicited by an effective vaccine strategy. Both T cells and antibody contribute to effective protection elicited by a highly lytic latency-deficient virus, AC-RTA. Importantly, our study reveals a previously unrecognized role of antibodies in controlling viral infection of the brain. However, whereas antibodies prevent WT virus from accessing the brain, AC-RTA can readily access the brain regardless of the presence of antibodies. AC-RTA was initially constructed to be a non-persistent virus by replacing the latency locus with an ectopic RTA expression cassette under the control of a strong promoter as a vaccine strategy for γ-herpesviruses. The unanticipated complications associated with AC-RTA infection in antibody-deficient mice underscore the importance of the mouse in vivo infection model for studying vaccination with live-attenuated mutant viruses.
Acknowledgements
We thank Meghan K. Jensen for excellent technical assistance and Drs. Jacob E. Kohlmeier and William W. Reiley for critical reading of the manuscript.
Footnotes
This project was funded by NIH grants AI084327 (to M.L.F.), AI049823 (to D.L.W.), AI042927 and AI082919 (to M.A.B.), CA148250 (to M.A.B. and R.S.), and DE019085 (to R.S.), and funds from the Trudeau Institute.
References
- 1.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 2.Wu TT, Blackman MA, Sun R. Prospects of a novel vaccination strategy for human gamma-herpesviruses. Immunol. Res. 2010;48:122–146. doi: 10.1007/s12026-010-8172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rickinson AB, Kieff E. Epstein-Barr Virus. In: Howley PM, Knipe DM, editors. Fields Virology. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 2575–2627. [Google Scholar]
- 4.Virgin HW, Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nash AA, Dutia BM, Stewart JP, Davison AJ. Natural history of murine gammaherpesvirus infection. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2001;356:569–579. doi: 10.1098/rstb.2000.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty PC, Christensen JP, Belz GT, Stevenson PG, Sangster MY. Dissecting the host response to a gamma-herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356:581–593. doi: 10.1098/rstb.2000.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Leonard P, Moreels A, Haumont M, Bollen A, Smets F, Denis M. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis. 2007;196:1749–1753. doi: 10.1086/523813. [DOI] [PubMed] [Google Scholar]
- 8.Stewart JP, Micali N, Usherwood EJ, Bonina L, Nash AA. Murine gamma-herpesvirus 68 glycoprotein 150 protects against virus- induced mononucleosis: a model system for gamma-herpesvirus vaccination. Vaccine. 1999;17:152–157. doi: 10.1016/s0264-410x(98)00190-x. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Flano E, Usherwood EJ, Surman S, Blackman MA, Woodland DL. Lytic cycle T cell epitopes are expressed in two distinct phases during MHV-68 infection. J. Immunol. 1999;163:868–874. [PubMed] [Google Scholar]
- 10.Stevenson PG, Belz GT, Castrucci MR, Altman JD, Doherty PC. A gamma-herpesvirus sneaks through a CD8(+) T cell response primed to a lytic-phase epitope. Proc. Nat. Acad. Sci. U.S.A. 1999;96:9281–9286. doi: 10.1073/pnas.96.16.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tibbetts SA, Loh J, Van Berkel V, McClellan JS, Jacoby MA, Kapadia SB, Speck SH, Virgin HW. Establishment and maintenance of gammaherpesvirus latency are independent of infective dose and route of infection. J. Virol. 2003;77:7696–7701. doi: 10.1128/JVI.77.13.7696-7701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman HM, de Lima B, Morton V, Stevenson PG. Murine gammaherpesvirus 68 lacking thymidine kinase shows severe attenuation of lytic cycle replication in vivo but still establishes latency. J. Virol. 2003;77:2410–2417. doi: 10.1128/JVI.77.4.2410-2417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flano E, Jia Q, Moore J, Woodland DL, Sun R, Blackman MA. Early Establishment of {gamma}-Herpesvirus Latency: Implications for Immune Control. J. Immunol. 2005;174:4972–4978. doi: 10.4049/jimmunol.174.8.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser JM, Farrell ML, Krug LT, Upton JW, Speck SH. A gammaherpesvirus 68 gene 50 null mutant establishes long-term latency in the lung but fails to vaccinate against a wild-type virus challenge. J. Virol. 2006;80:1592–1598. doi: 10.1128/JVI.80.3.1592-1598.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usherwood EJ, Roy DJ, Ward K, Surman SL, Dutia BM, Blackman MA, Stewart JP, Woodland DL. Control of Gammaherpesvirus Latency by Latent Antigen-specific CD8(+) T Cells. J. Exp. Med. 2000;192:943–952. doi: 10.1084/jem.192.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Usherwood EJ, Ward KA, Blackman MA, Stewart JP, Woodland DL. Latent antigen vaccination in a model gammaherpesvirus infection. J. Virol. 2001;75:8283–8288. doi: 10.1128/JVI.75.17.8283-8288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibbetts SA, McClellan JS, Gangappa S, Speck SH, Virgin HW. Effective Vaccination against Long-Term Gammaherpesvirus Latency. J. Virol. 2003;77:2522–2529. doi: 10.1128/JVI.77.4.2522-2529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayhan B, Yager EJ, Lanzer K, Cookenham T, Jia Q, Wu T-T, Woodland DL, Sun R, Blackman MA. A replication-deficient murine gamma-herpesvirus blocked in late viral gene expression can establish latency and elicit protective cellular immunity. J. Immunol. 2007;179:8392–8402. doi: 10.4049/jimmunol.179.12.8392. [DOI] [PubMed] [Google Scholar]
- 19.Tibbetts SA, Suarez F, Steed AL, Simmons JA, Virgin HW. A gamma-herpesvirus deficient in replication establishes chronic infection in vivo and is impervious to restriction by adaptive immune cells. Virology. 2006;353:210–219. doi: 10.1016/j.virol.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Boname JM, Coleman HM, May JS, Stevenson PG. Protection against wild-type murine gammaherpesvirus-68 latency by a latency-deficient mutant. J. Gen. Virol. 2004;85:131–135. doi: 10.1099/vir.0.19592-0. [DOI] [PubMed] [Google Scholar]
- 21.Fowler P, Efstathiou S. Vaccine potential of a murine gammaherpesvirus-68 mutant deficient for ORF73. J. Gen. Virol. 2004;85:609–613. doi: 10.1099/vir.0.19760-0. [DOI] [PubMed] [Google Scholar]
- 22.Rickabaugh TM, Brown HJ, Martinez-Guzman D, Wu TT, Tong L, Yu F, Cole S, Sun R. Generation of a latency-deficient gammaherpesvirus that is protective against secondary infection. J. Virol. 2004;78:9215–9223. doi: 10.1128/JVI.78.17.9215-9223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia Q, Freeman ML, Yager EJ, McHardy I, Tong L, Martinez-Guzman D, Rickabaugh T, Hwang S, Blackman MA, Sun R, Wu TT. Induction of protective immunity against murine gammaherpesvirus 68 infection in the absence of viral latency. J. Virol. 2010;84:2453–2465. doi: 10.1128/JVI.01543-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman ML, Lanzer KG, Cookenham T, Peters B, Sidney J, Wu TT, Sun R, Woodland DL, Sette A, Blackman MA. Two Kinetic Patterns of Epitope-Specific CD8 T-Cell Responses following Murine Gammaherpesvirus 68 Infection. J. Virol. 2010;84:2881–2892. doi: 10.1128/JVI.02229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim IJ, Flano E, Woodland DL, Blackman MA. Antibody-mediated control of persistent gamma-herpesvirus infection. J. Immunol. 2002;168:3958–3964. doi: 10.4049/jimmunol.168.8.3958. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson PG, Doherty PC. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J. Virol. 1998;72:943–949. doi: 10.1128/jvi.72.2.943-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obar JJ, Fuse S, Leung EK, Bellfy SC, Usherwood EJ. Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J. Virol. 2006;80:8303–8315. doi: 10.1128/JVI.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yager EJ, Kim IJ, Freeman ML, Lanzer KG, Burkum CE, Cookenham T, Woodland DL, Blackman MA. Differential impact of ageing on cellular and humoral immunity to a persistent murine gamma-herpesvirus. Immun Ageing. 2010;7:3. doi: 10.1186/1742-4933-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumazaki K, Tirosh B, Maehr R, Boes M, Honjo T, Ploegh HL. AID−/− mus−/− mice are agammaglobulinemic and fail to maintain B220-CD138+ plasma cells. J. Immunol. 2007;178:2192–2203. doi: 10.4049/jimmunol.178.4.2192. [DOI] [PubMed] [Google Scholar]
- 31.Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J. Immunol. 2008;181:4168–4176. doi: 10.4049/jimmunol.181.6.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flano E, Husain SM, Sample JT, Woodland DL, Blackman MA. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 2000;165:1074–1081. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- 33.Flano E, Kim IJ, Woodland DL, Blackman MA. gamma-Herpesvirus Latency Is Preferentially Maintained in Splenic Germinal Center and Memory B Cells. J. Exp. Med. 2002;196:1363–1372. doi: 10.1084/jem.20020890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 35.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 36.Terry LA, Stewart JP, Nash AA, Fazakerley JK. Murine gammaherpesvirus-68 infection of and persistence in the central nervous system. J. Gen. Virol. 2000;81:2635–2643. doi: 10.1099/0022-1317-81-11-2635. [DOI] [PubMed] [Google Scholar]
- 37.Cho HJ, Kim S, Kwak SE, Kang TC, Kim HS, Kwon HJ, Kim YW, Kim YS, Choi EK, Song MJ. Age-dependent pathogenesis of murine gammaherpesvirus 68 infection of the central nervous system. Mol Cells. 2009;27:105–111. doi: 10.1007/s10059-009-0011-5. [DOI] [PubMed] [Google Scholar]
- 38.Hausler M, Sellhaus B, Scheithauer S, Engler M, Alberg E, Teubner A, Ritter K, Kleines M. Murine gammaherpesvirus-68 infection of mice: A new model for human cerebral Epstein-Barr virus infection. Ann Neurol. 2005;57:600–603. doi: 10.1002/ana.20440. [DOI] [PubMed] [Google Scholar]
- 39.Morrison LA, Knipe DM. Contributions of antibody and T cell subsets to protection elicited by immunization with a replication-defective mutant of herpes simplex virus type 1. Virology. 1997;239:315–326. doi: 10.1006/viro.1997.8884. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson PG, Cardin RD, Christensen JP, Doherty PC. Immunological control of a murine gammaherpesvirus independent of CD8+ T cells. J. Gen. Virol. 1999;80:477–483. doi: 10.1099/0022-1317-80-2-477. [DOI] [PubMed] [Google Scholar]
- 41.Gulley ML, Tang W. Laboratory assays for Epstein-Barr virus-related disease. J Mol Diagn. 2008;10:279–292. doi: 10.2353/jmoldx.2008.080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Carlo P, Trizzino M, Titone L, Capra G, Colletti P, Mazzola G, Pistoia D, Sarno C. Unusual MRI findings in an immunocompetent patient with EBV encephalitis: a case report. BMC Med Imaging. 2011;11:6. doi: 10.1186/1471-2342-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volpi A. Epstein-Barr virus and human herpesvirus type 8 infections of the central nervous system. Herpes. 2004;11(Suppl 2):120A–127A. [PubMed] [Google Scholar]
- 44.Chan PK, Ng HK, Cheung JL, Cheng AF. Survey for the presence and distribution of human herpesvirus 8 in healthy brain. J Clin Microbiol. 2000;38:2772–2773. doi: 10.1128/jcm.38.7.2772-2773.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapadia SB, Levine B, Speck SH, Virgin HW. Critical role of complement and viral evasion of complement in acute, persistent, and latent gamma-herpesvirus infection. Immunity. 2002;17:143–155. doi: 10.1016/s1074-7613(02)00369-2. [DOI] [PubMed] [Google Scholar]
- 46.Kapadia SB, Molina H, van Berkel V, Speck SH, Virgin HW. Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J. Virol. 1999;73:7658–7670. doi: 10.1128/jvi.73.9.7658-7670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarakanova VL, Molleston JM, Goodwin M, Virgin HW. MHV68 complement regulatory protein facilitates MHV68 replication in primary macrophages in a complement independent manner. Virology. 2010;396:323–328. doi: 10.1016/j.virol.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weck KE, Kim SS, Virgin HW, Speck SH. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]