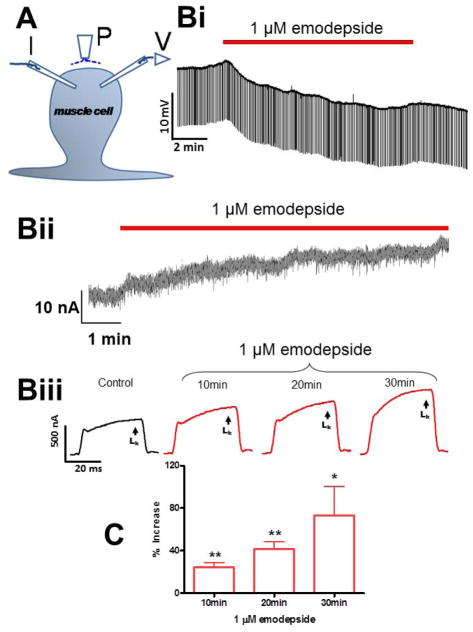
Figure 3.
Electrophysiological techniques (two micropipette current-clamp and voltage-clamps) for recording from Ascaris suum. (A) A. suum muscle bag showing the current (I) and voltage (V) micropipettes in the bag, and the perfusion needle (P). (Bi) Representative current-clamp traces showing the slow hyperpolarizing membrane potential during and after 10 min application of 1 μM emodepside. Note that the trace does not get thinner because the membrane resistance does not change detectably. (Bii) Outward current response to 1 μM emodepside at higher time resolution. Holding potential −35 mV. Notice that emodepside produces a gradually increasing current after a delay of some 30 seconds. The response does not plateau in the time period of this recording. (B III) voltage-clamp traces of control K current and the time-dependent effects of 1 μM emodepside on the K currents, all to a step potential of 0 mV from a holding potential of −35 mV. (C) Bar chart (mean ± s.e.) of 1 μM emodepside effect on steady state (LK) currents. Comparison was made between the control 0 mV step current at 30 – 40 ms and the corresponding current increased by emodepside at 10, 20 and 30 min. Emodepside increased LK currents at 10 min (p < 0.01, n = 4, paired t-test), 20 min (p < 0.01, n = 4, paired t-test) and 30 min (p < 0.05, n = 4, paired t-test). Figure, modified from Buxton et al. (2011).