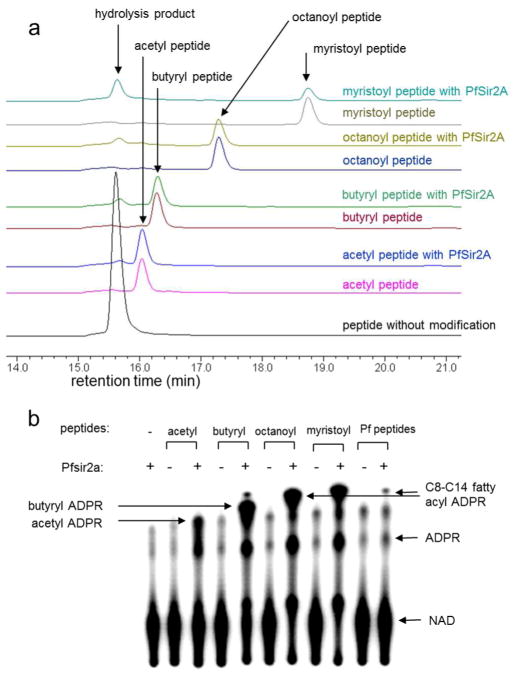
Figure 1.
PfSir2A could hydrolyze medium and long chain fatty acyl lysine more efficiently than acetyl lysine. a) Overlaid HPLC traces showing PfSir2A-catalyzed hydrolysis of different fatty acyl lysine peptides. Acyl peptides were used at 20 μM, PfSir2A at 1 μM, and NAD at 500 μM. The corresponding synthetic peptide without any acyl lysine modification (H3K9WW unmodified) was used as the control to indicate the position of the hydrolysis product formed. b) 32P-NAD assay could detect the presence of medium or long chain fatty acyl lysine modifications on P. falciparum proteins. PfSir2A were incubated with 32P-NAD and synthetic peptides bearing different acyl modifications. Negative controls were reactions without PfSir2A or peptides. The reactions were resolved by TLC and detected by autoradiography. With P. falciparum peptides (last two lanes), the acyl ADPR spot formed was similar to the C8–C14 acyl ADPR, suggesting that such fatty acyl groups were present and could be removed by PfSir2A.