Table 1.
Potency (EC50) and maximal stimulation (Emax)s of hCB1 and hCB2 by compounds 13–32.[a]
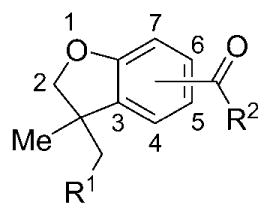 | |||||||
|---|---|---|---|---|---|---|---|
| Compd | R1 | R2 | R2 position | γ-[35S]GTP hCB1 | γ-[35S]GTP hCB2 | ||
| EC50 [nm ± SEM] | Emax [%][b] | EC50 [nm ± SEM] | Emax [%][b] | ||||
| 13 | phenyl | N-(2-iodophenyl) | 5 | >10 000 | ND[c] | >10 000 | ND |
| 14 | phenyl | N-cyclohexyl | 5 | >10 000 | ND | 406 ± 1.4 | 47.1 |
| 15 | phenyl | 1-piperidyl | 5 | >10 000 | ND | >10 000 | ND |
| 16 | phenyl | N-(2-iodophenyl) | 6 | >10 000 | ND | >10 000 | ND |
| 17 | phenyl | N-cyclohexyl | 6 | >10 000 | ND | 478 ± 1.3 | 54.3 |
| 18 | phenyl | 1-piperidyl | 6 | >10 000 | ND | 128 ± 32 | 88.3 |
| 33 | S enantiomer (1) of 18 | 6 | >10 000 | ND | 108.02 | 86 | |
| 34 | R enantiomer (2) of 18 | 6 | >10 000 | ND | 960.89 | 43.3 | |
| 19 |
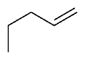
|
1-piperidyl | 6 | 856 ± 1.2 | 60 | 48.9± 1.4 | 97.1 |
| 20 |
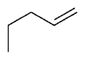
|
N-cyclohexyl | 6 | >10 000 | ND | 839 ± 5.5 | 105 |
| 21 | phenyl |
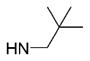
|
6 | >10 000 | ND | 246 ± 1.3 | 48.7 |
| 22 | phenyl | morpholine | 6 | >10 000 | ND | 5583 ± 4.4 | 93 |
| 23 | phenyl |
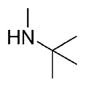
|
6 | 2580 ± 1.5 | ND | 95.3±1.8 | 91 |
| 24 | phenyl |
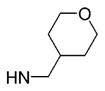
|
6 | >10 000 | ND | 659 ±1.4 | 49 |
| 27 | 1-naphthyl | N-cyclohexyl | 6 | >10 000 | ND | >10 000 | ND |
| 28 | 1-naphthyl | 1-piperidyl | 6 | >10 000 | ND | >10 000 | ND |
| 29 | 2-naphthyl | 1-piperidyl | 6 | >10 000 | ND | 875 ± 1.4 | 112 |
| 30 | 4-chlorohenyl | 1-piperidyl | 6 | 1363 ± 1.4 | 86.9 | 56.2± 1.2 | 102 |
| 31 | 4-methoxyphenyl | 1-piperidyl | 6 | >10 000 | ND | 234.68 | 77.1 |
| 32 | 4-pyridine | 1-piperidyl | 6 | >10 000 | ND | 2801.44 | 62.5 |
CB1 and CB2 assay data are presented as the mean of two determinations; reproducibility was monitored by the use of compound 7 as reference. For replicate determinations, the maximum variability tolerated in the test was ±20 % around the average of the replicates.
Efficacies for CB1 or CB2 are expressed as percentage relative to the efficacy of compound 7.
ND =not determined (plateau was not reached at a dose of 10 μm).
